Abstract
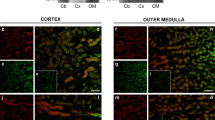
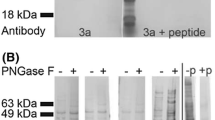
Aquaporin CHIP, a 28 kDa channel forming protein, has been proposed to function as water channel in both erythrocyte and kidney proximal tubule. Recently, we have reported that in frog urinary bladder, a model of the kidney collecting tubule, polyclonal antibodies against human erythrocyte CHIP recognize and immunoprecipitate a 30 kDa protein from the epithelial cell homogenate. In the present work confocal fluorescence microscopy was used to determine the cellular and subcellular localization of CHIP28-like proteins in the urinary epithelium. A clear labeling of the apical border was found after Triton X-100 permeabilization. The labeling was distributed throughout the apical domain and not restricted to specific domains of the membrane. The staining was also present in the deeper confocal sections where the fluorescence seems to be localized at the cellular contour. No difference in the labeling patterns was observed between resting and ADH-treated bladder. Specificity of the staining was confirmed by the absence of the labeling pattern when antiserum was preadsorbed on CHIP28 protein immobilized on Immobilon P stripes. Our results suggest that CHIP-like proteins are not proteins inserted in the apical membrane during the antidiuretic response. Moreover, we do not know whether the labeling was due to the presence of CHIP28 itself or an as-yet-unidentified protein sharing immunological analogies with aquaporin CHIP.
Similar content being viewed by others
References
Abrami, L. Simon, M., Rousselet, G., Berthonaud, V., Buhler, J.M., Ripoche, P. 1994. Sequence and functional expression of an amphibian water channel, FA-CHIP: a new member of the MIP family. Biochim. Blophys. Acta 1192:147–151
Agre, P., Sasaki, S., Chrispeels, M.J. 1993. Aquaporins: a family of water channel proteins. Am. J. Physiol. 265:F461
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72:248–254
Calamita, G., Mola, M.G., Svelto, M. 1994. Presence in frog urinary bladder of protein immunologically related to the aquaporin-CHIP. Eur. J. Cell. Biol. 64:222–228
Chevalier, J., Bourguet, J., Hugon, J.S. 1974. Membrane associated particles: distribution in frog urinary bladder epithelium at rest and after oxytocin treatment. Cell Tissue Res. 152:129–140
Denker, B.M., Smith, B.L., Kuhajda, R.P., Agre, P. 1988. Identification, purification and partial characterization of a novel Mr 28000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 263:15634–15642
Fairbanks, G., Steck, T.L., Wallach, D.F.H. 1971. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10:2606–2616
Fushimi, K., Uchida, S., Hara, Y., Hirata, Y., Marumo, F., Sasaki 1993. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361:549–552
Handler, J.S., Orloff, J. 1973. The mechanism of action of antidiuretic hormone. In: Handbook of Physiology, Renal Physiology. Sect. 8, Chapter 24, pp. 791–814. Am. Physiol. Soc., Washington, DC
Harmanci, M.C., Stern, P., Kachadorian, W.A., Valtin, H., DiScala, V.A. 1980. Vasopressin and collecting duct intramembranous particle clusters: a dose response relationship. Am. J. Physiol. 239:F560-F564
Hasegawa, H., Zhang, R., Dohrman, A., Verkman, A.S. 1993. Tissue specific expression of mRNA encoding rat kidney water channel CHIP28 by in situ hybridization. Am. J. Physiol. 264: C237-C245
Hebert, S.C., Andreoli, T.E. 1982. Water movement across the mammalian cortical collecting duct. Kidney Int. 22:526–535
Kachadorian, W.A., Wade, J.B., DiScala, V.A. 1975. Vasopressin: induced structural change in toad bladder luminal membrane. Science 190:67–69
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680–685
Nielsen, S., DiGiovanni, S., Christensen, E.I., Knepper, M.A., Harris, H.W. 1993. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc. Natl. Acad. Sci. USA 90:11663–11667
Nielsen, S., Smith, B.L., Christensen, E.I., Knepper, M.A., Agre, P. 1993. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J. Cell. Biol. 2120:371–383
Nielsen, S., Smith, B.L., Christensen, E.I., Knepper, M.A., Agre, P. 1993. Distribution of the aquaporin CHIP in secretory and re-absorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 90:7275–7279
Sabolic, I., Valenti, G., Verbavatz, J.M., Van Hoeck, A., Verkman, A.S., Ausiello, D., Brown, D. 1992. Localization of the CHIP28 water channel in rat kidney. Am. J. Physiol. 263:C1225-C1233
Smith, B.L., Agre, P. 1991. Erythrocyte Mr 28000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J. Biol. Chem. 266:6407–6415
Van Der Goot, F., Corman, B., Ripoche, P. 1991. Evidence for permanent water channels in the basolateral membrane of an ADH-sensitive epithelium. J. Membrane Biol. 120:59–65
Verbavatz, J.M., Frigeri, A., Gobin, R., Ripoche, P., Bourguet, J. 1992. Effect of salt acclimation on water and urea permeabilities across the frog bladder: relationship with intramembrane particle aggregates. Comp. Biochem. Physiol. 101:827–833
Wade, J.B., Stetson, D.L., Lewis, S.A. 1981. ADH action: Evidence for a membrane shuttle hypothesis. Ann. NY Acad. Sci. 372:106–107
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Calamita, G., Mola, M.G., Gounon, P. et al. Aquaporin-CHIP-related protein in frog urinary bladder: Localization by confocal microscopy. J. Membarin Biol. 143, 267–271 (1995). https://doi.org/10.1007/BF00233455
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00233455