Abstract
Water transfer by osmosis through pores occurs either by viscous flow or diffusion depending on whether the driving osmolyte is able to enter the pore. Analysis of osmotic permeabilities (P os )measured in antibiotic and cellular pore systems supports this distinction, showing that P os approaches either the viscous value (P f ) or the diffusive value (P d )depending on the size of the osmolyte in relation to the pore radius. Macroscopic hydrodynamics and diffusion theory, when used with drag and steric coefficients within an appropriate osmotic model, apply with remarkable accuracy to channels of molecular dimensions where water molecules cannot pass each other, without the need to postulate any special flow regimes.
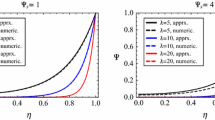
It becomes apparent that the true viscous to diffusive flow ratio, P f /P d , can be separated from the effects of tracer filing by osmotic measurements alone. It does not monotonically decrease with the pore radius but rises steeply at the smaller radii which would apply to pores in cell membranes. Consequently, the application of the theory to osmotic and diffusive flow data for the red cell predicts a pore radius of 0.2 nm in agreement with other recent measurements on isolated components of the system, showing that the viscous-diffusive distinction applies even in molecular pores.
Similar content being viewed by others
References
Agre, P., Preston, G.M. 1991. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: Member of an ancient channel family. Proc. Natl. Acad. Sci. USA 88:11110–11114
Andreoli, T.E., Dennis, V.W., Weigl, A.M. 1969. The effect of Amphotericin B on the water and non-electrolyte permeability of thin lipid membranes. J. Gen. Physiol. 53:133–156.
Beck, R.E., Schultz, J.S. 1972. Hindrance of solute diffusion within membranes as measured with microporous membranes of known pore geometry. Biochim. Biophys. Acta 255:273–303
Castillo, L.F., Mason, E.A. 1980. Statistical-mechanical theory of passive transport through partially sieving or leaky membranes. Biophys. Chem. 12:223–233
Cohen, B.E. 1975. The permeability of liposomes to non-electrolytes: I. Activation energies for permeation. J. Membrane Biol. 20:205–234
Dainty, J., Ginzburg, B.Z. 1963. Irreversible thermodynamics and frictional models of membrane processes. J. Theor. Biol. 5:256–265
Dani, J.A., Levitt, D.G. 1981. Binding constants for Li, K, and T1, in the gramicidin channel determined from water permeability measurements. Biophys. J. 35:485–500
Durbin, R.P. 1960. Osmotic flow of water across permeable cellulose membranes. J. Gen. Physiol. 44:315–326
Durbin, R.P., Frank, H., Solomon, A.K. 1956. Water flow through frog gastric mucosa. J. Gen. Physiol. 39:535–551
Faxen, H. 1922. Die Bewegung einer starren kugel längs der Achse eines mit zäher Flussigkeit gefullten Rohres. Archiv for Matematik, Astronomi och Fysik. Band 17 27:1–28
Faxen, H. 1922. Der Widerstand gegen die Bewegung einer starren Kugel in einer zahen Flussigkeit, die zwischen zwei parallelen ebenen Wanden eingeschlossen ist. Annalen der Physik 68:89–119
Ferry, J.D. 1936. Statistical evaluation of sieve constants in ultrafiltration. J. Gen. Physiol. 20:95–104
Finkelstein, A. 1974. Aqueous pores created in thin lipid membranes by the antibiotics nystatin, amphotericin B and gramicidin A: Implications for pores in plasma membranes. In: Drugs and Transport Processes. B.A. Callingham, editor, pp. 241–250. MacMillan, London
Finkelstein, A. 1987. Water Movement through Lipid Bilayers, Pores and Plasma Membranes. John Wiley, New York
Fischbarg, J., Kuang, K., Li, J., Arant-Hickman, S., Vera, J., Loike, J. 1993. Facilitative and sodium-dependent glucose transporters behave as water channels. In: Isotonic Transport in Leaky Epithelia. H.H. Ussing, J. Fischbarg, O. Sten-Knudsen, E.H. Larsen, N.J. Willumsen, editors, pp. 432–446. Munksgaard, Copenhagen
Galey, W.R., Brahm, J. 1985. The failure of hydrodynamic analysis to define pore size in cell membranes. Biochim. Biophys. Acta 818:425–428
Ginzburg, B.Z., Katchalsky, A. 1963. The frictional coefficients of the flows of nonelectrolytes through artificial membranes. J. Gen. Physiol. 47:403–418
Haberman, W.L., Sayre, R.M. (1958). Motions of rigid and fluid spheres in stationary and moving liquids inside cylindrical tubes. David Taylor Model Basin. US Dept. Navy Report No. 1143
Hill, A.E. 1982. Osmosis: a bimodal theory with implications for symmetry. Proc. Roy. Soc. Lond. B. 215:155–174
Hill, A.E. 1989. Osmosis in leaky pores: the role of pressure. Proc. R. Soc. Lond. B. 237:363–367
Hill, A.E. 1989. Osmotic flow equations for leaky porous membranes. Proc. Roy. Soc. Lond. B. 237:369–377
Hodgkin, A.L., Keynes, R.D. 1955. Active transport of cations in giant axons from Sepia and Loligo. J. Physiol. 128:28–60
Holz, R., Finkelstein, A. 1970. The water and nonelectrolyte permeability induced in thin lipid membranes by the polyene antibiotics Nystatin and Amphotericin B. J. Gen. Physiol. 56:125–145
Kedem, O., Katchalsky, A. 1961. A physical interpretation of the phenomenological coefficients of membrane permeability. J. Gen. Physiol. 45:143–179
Levitt, D.G. 1973. Kinetics of diffusion and convection in 3.2 A pores. Biophys. J. 13:186–206
Levitt, D.G., Elias, S.R., Hautman, J.M. 1978. Number of water molecules coupled to the transport of sodium, potassium and hydrogen ions via gramicidin, onactin or valinomycin. Biochim. Biophys. Acta 512:436–451
Longuet-Higgins, H.C., Austin, G. 1966. The kinetic of osmotic transport through pores of molecular dimensions. Biophys. J. 6:217–224
Mauro, A. 1957. Nature of solvent transfer in osmosis. Science 126:252–253
Moura, T.F., Macey, R.I., Chien, D.Y., Karan, D., Santos, H. 1984. Thermodynamics of all-or-none water channel closure in red cells. J. Membrane Biol. 81:105–111
Preston, G.M., Carroll, T.P., Guggino, W.B., Agre, P. 1992. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
Renkin, E.M. 1954. Filtration, diffusion and molecular sieving through porous cellular membranes. J. Gen. Physiol. 38:225–243
Renkin, E.M., Curry, F.E. 1979. Chapter 1. Transport of water and solutes across capillary endothelium. In: Membrane Transport in Biology, Vol IVA. G. Giebisch, D.C. Tosteson, H.H. Ussing, editors, pp. 1–45. Springer, New York
Rosenberg, P.A., Finkelstein, A. 1978. Water permeability of gramicidin A-treated lipid bilayer membranes. J. Gen. Physiol. 72:341–350
Solomon, A.K. 1968. Characterization of biological membranes by equivalent pores. J. Gen. Physiol. 51:335s-364s
van Hoek, A.N., Verkman, A.S. 1992. Functional reconstitution of the isolated erythrocyte water channel CHIP28. J. Biol. Chem. 267:18267–18269
Vegard, L. 1908. On the free pressure in osmosis. Proc. Camb. Phil. Soc. 15:13–23
Verkman, A.S. 1992. Water channels in cell membranes. Annu. Rev. Physiol. 54:97–108
Wang, H., Skalak, R. 1969. Viscous flow in a cylindrical tube containing a line of spherical particles. J. Fluid Mech. 38:75–96
Whittembury, G., Carpi-Medina, P. 1988. Renal absorption of water: are there pores in proximal tubule cells? NIPS 3:61–65
Author information
Authors and Affiliations
Additional information
I should like to thank Drs. B. and Y.Y. Shachar-Hill for many helpful discussions on this problem and for their time and effort expended in critically reading the manuscript at numerous stages. This paper originated as an invited talk given to the annual meeting on Membrane Transport at Sandbjerg, Denmark in June 1992.
Rights and permissions
About this article
Cite this article
Hill, A.E. Osmotic flow in membrane pores of molecular size. J. Membarin Biol. 137, 197–203 (1994). https://doi.org/10.1007/BF00232588
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00232588