Abstract
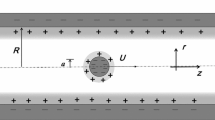
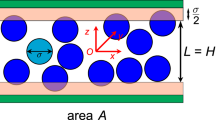
Diffusion is fundamental to the random movement of solutes in solution throughout biological systems. Theoretical studies of diffusing solutes across cell membranes confined in a microscopic size of pores have been an interesting subject in life and medical sciences. When a solute is confined in a critical area of membrane pores, which shows a quite different behavior compared to the homogeneous-bulk fluid whose transport is isotropic in all directions. This property has novel features, which are of considerable physiological interest.


Similar content being viewed by others
Change history
08 November 2017
The original version of this article unfortunately contained two mistakes.
References
Benz R (2006) Bacterial and eukaryotic Porins. Structure, function, mechanism. Wiley-VCH, Weinheim
Boal D (2002) Mechanics of the cell. Cambridge University Press, Cambridge
Duclohier H (2009) Structure-function studies on the voltage-gated sodium channel. Biochim Biophys Acta 1788:2374–2379
Dutzler R (2007) A structural perspective on CIC channel and transporter function. FEBS Lett 581(15):2839–2844
Fioroni MD, Woreck T, Rodriguez-Ropero F (2013) β-Barrel channel proteins as tools in nanotechnology: biology, basic science and advanced applications. Springer, Berlin
Fradin C, Satsoura D, Andrews DW (2009) Punching holes in membranes: how oligomeric pore-forming proteins and lipids cooperate to form aqueous channels in membranes. Handbook of Modern Biophysics 2:223–262
Frank HYU, Catterall WA (2003) Overview of the voltage-gated sodium channel family. Genome Biol 4:207
Hilitski F, Ward AR, Cajamarca L, Hagan MF, Grason GM, Dogic Z (2015) Measuring cohesion between macromolecular filaments one pair at a time: depletion-induced microtubule bundling. Phys Rev Lett 114:138102
Iglic A, Kralj-Iglic V, Drobne D (2015) Nanostructures in biological systems: theory and applications. CRC Press, Taylor & Francis, Boca Raton and Abingdon
Israelachvili J (2002) Intermolecular surface forces, 2nd edn. Academic Press, Cambridge
Kozlov MM, Chernomordik LV (2015) Membrane tension and membrane fusion. Curr Opin Struct Biol 33:61–67
Liu WM (1998) Shear numbers of protein β-barrels: definition, refinements and statistics. J Mol Biol 275:541–545
Marban E, Yamagishi T, Tomaselli GF (1998) Structure and function of voltage-gated sodium channels. J Physiol 508(3):647–657
Mouritsen OG, Bagatolli LA (2016) Life- as a matter of fat: lipids in a membrane biophysics perspective, 2nd edn. Springer International Publishing, Switzerland
Noskov SY, Bernéche S, Roux B (2004) Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands. Nature 43(7010):830–834
Ollia S, Vattulainen I (2010) Lateral precision profiles in lipid membranes: dependence on molecular composition. Molecular simulations and biomembranes: for biophysics to function (Royal Society of Chemistry). Invited review 1:26–55
Perozo E, Kloda A, Cortes DM, Martina B (2002) Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Mol Biol 9:696–703
Phillips R, Kondev J, Theriot J, Gercia HG, Orme N (2012) Physical bioology of the cell (2nd Ed). Garland Science, Taylor & Francis, Abingdon
Portet T, Dimova R (2010) A new method for measuring edge tensions and stability of lipid bilayesrs: effect of membrane composition. Biophys J 99:3264–3273
Quick MW (2003) Transmembrane transporters. John Wiley & Sons, Inc., Hoboken
Rawicz W, Olbrich KC, McIntosh T, Nedham D, Evans E (2000) Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J 79(1):328–339
Simunovic M, Voth GA (2015) Membrane tension controls the assembly of curvature-generating proteins. Nat Commun 6:7219
Soveral G, Nielsen S, Casini A (2015) Aquaporins in health and disease: new molecular targets for drug discovery. CRC Press, Taylor & Francis, Boca Raton and Abingdon
Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O’Connell J, Stroud RM, Schulten K (2002) Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 296(5567):525–530
Vial F, Oukhaled AG, Auvray L, Tribet C (2006) Long-living channels of well defined radius opened in lipid bilayers by polydisperse, hydrophobically-modified polyacrylic acids. Soft Matter 3:75–78
Zheng J, Trudeau MC (2016) Handbook of ion channels. CRC Press, Taylor & Francis, Boca Raton and Abingdon
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: The Greek capital letter Theta is missing in the PDF version in the sentence “Where the two-order in Θ represents a stable pore with a critical pore area Δ=π 2/4.” should be corrected as “Where the two-order in Θ represents a stable pore with a critical pore area Δ=πΘ2/4.” A word was also misspelled “legands” should be “ligands”.
A correction to this article is available online at https://doi.org/10.1007/s10863-017-9731-y.
Rights and permissions
About this article
Cite this article
Haque, M.M. Diffusion coefficient in biomembrane critical pores. J Bioenerg Biomembr 49, 445–450 (2017). https://doi.org/10.1007/s10863-017-9726-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-017-9726-8