Abstract
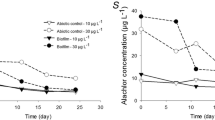
A static microcosm system was used to evaluate effects of the herbicide diquat (0.3–30 mg/L) on the structure and function of naturally derived microbial communities on polyurethane foam substrates. Microbial communities were exposed to a single application of diquat and were monitored for 21 days. Effects on community structure included changes in algal cell density at diquat concentrations ⩾0.3 mg/L (after an initial decrease in net productivity), bacterial cell density (1 mg/L diquat), and increased biomass accumulation (10 and 30 mg/L diquat). The species richness of protozoa was reduced at diquat concentrations >0.3 mg/L; protozoan species composition was progressively more dissimilar with diquat treatment. Effects on community function included inhibition of net productivity and community respiration (10 and 30 mg/L diquat), and decreased enzyme activities [alkaline phosphatase (1, 10, and 30 mg/L diquat), electron transport system (⩾0.3 mg/L diquat), and β-glucosidase (⩾0.3 mg/L diquat)]. Both photosynthetic and nonphotosynthetic organisms were affected by diquat. Most structural and functional responses were sensitive indicators of stress. Estimated chronic toxicity values ranged from 0.3 mg/L (day 3) to 5.5 mg/L (day 21). Most microbial responses indicated that microbial community structure and function did not recover within the 21-day exposure period.
Similar content being viewed by others
References
Birmingham BC, Colman B (1983) Potential phytotoxicity of diquat accumulated by aquatic plants and sediments. Water Air Soil Pollut 19:123–131
Blanck H, Wängberg S-A, Molander S (1988) Pollution-induced community tolerance—a new ecotoxicological tool. In: Cairns J Jr, Pratt JR (eds), Functional testing of aquatic biota for estimating hazards of chemicals. ASTM, Philadelphia, pp 219–230
Bradford MM (1976) A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem 72:248–254
Breazeale FW, Camper ND (1972) Effect of selected herbicides on bacterial growth rates. Appl Microbiol 23:431–432
Cairns, J Jr, Kuhn DL, and Plafkin JL (1979) Protozoan colonization of artificial substrates. In: Weitzel RL (ed), Methods and measurement of periphyton communities: A review. ASTM, Philadelphia, pp 39–54
Chrost RJ (1990) Microbial ectoenzymes in aquatic environments. In: Overbeck J, Chrost RJ (eds), Aquatic microbial ecology. Springer-Verlag, NY, pp 47–74
— (1989) Characterization and significance of β-glucosidase activity in lake water. Limnol Oceanogr 34:660–672
Cragg BA, Fry JC (1984) The use of microcosms to simulate field experiments to determine the effects of herbicides on aquatic bacteria. J Gen Microbiol 130:2309–2316
Cullimore DR (1975) The in vivo sensitivity of some species of Chlorophyceae to a selected range of herbicides. Weed Res 15:401–406
Dunnett CW (1955) Multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50:1096–1121
Ferebee RN, Guthrie RK (1973) The effects of selected herbicides on bacterial populations in an aquatic environment. Water Res Bull 9:1125–1135
Ganstad EO (1982) Weed control methods for recreational facilities management. CRC Press, Boca Raton, FL
Jager G (1983) Herbicides. In: Buchel KH (ed), Chemistry of pesticides. John Wiley & Sons, NY, pp 322–392
Johnson TB (1986) Potential impact of selected agricultural chemical contaminants on northern prairie wetland: A microcosm evaluation. Environ Toxicol Chem 5:473–485
JT Baker (1984) Baker-10SPE Application Guide. Vol. II. JT Baker Chemical Co, Philipsburg, NJ
McConnell W (1962) Productivity relations in carboy microcosms. Limnol Oceanogr 7:335–343
Morrison SJ, King JD, Bechtold RE, White DC (1977) Evidence for microfloral succession on allochthonous plant litter in Apalachicola Bay, Florida, USA. Mar Biol 41:229–240
Odum, EP (1985) Trends expected in stressed ecosystems. BioScience 35:419–422
Philips EJ, Hansen P, and Verlardi T (1992) Effects of the herbicide diquat on the growth of microalgae and cyanobacteria. Bull Environ Contam Toxicol 49:750–756
Porter KG, Feig Y (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Pratt JR, Bowers NJ, Cairns J Jr (1990) Effects of sediment on estimates of diquat toxicity in laboratory microcosms. Water Res 24:51–57
Pratt JR, Smith EP (1991) Significance of change in community structure: A new method for testing differences. In: Biological criteria: Research and regulation, EPA 440/5–91–005. US Environmental Protection Agency, Washington, DC, pp 91–103
Rausch P (1981) The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia 78:237–251
Sayler GS, Puziss M, Silver M (1979) Alkaline phosphatase assay for freshwater sediments: application to perturbed sediment systems. Appl Envir Microbiol 38:922–927
Simsiman GV, Daniel TC, Chesters G (1976) Diquat and endothall: Their fates in the environment. Res Rev 62:132–174
Sokal RR, Rohlf FJ (1983) Biometry. WH Freeman, NY
Stokes DM, Turner JS (1971) The effects of the dipyridyl diquat on the metabolism of Chlorella vulgaris; III. Dark metabolism. Effects on respiration rate and the path of carbon. Aust J Biol Sci 24:433–447
Stumm W, Morgan JJ (1981) Aquatic Chemistry: An introduction emphasizing chemical equilibria in natural waters, 2nd ed. John Wiley & Sons Inc, NY
Summers LA (1980) The bipyridinium herbicides. Academic Press, London
Turner JS, Stokes DM, Gilmore LB (1970) The effects of the dipyridyl diquat on the metabolism of Chlorella vulgaris I. Gas exchange in the light. Aust J Biol Sci 23:43–61
Van Rensen JJS (1969) Effects of diquat on photosynthesis in Scenedesmus. Meded Landbouwhogesch Wageningen 69-4:1–11
Velji MI, Albright LJ (1986) Microscopic enumeration of attached marine bacteria of seawater, marine sediment, fecal matter and kelp blade samples following pyrophosphate and ultrasound treatments. Can J Microbiol 32:121–126
Wallnoefer VP (1968) Untersuchungen über die antimikrobielle Wirkung von Deiquat und Paraquat. Z Pflanzenkr Pflanzenschutz 75:218–224
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Melendez, A.L., Kepner, R.L., Balczon, J.M. et al. Effects of diquat on freshwater microbial communities. Arch. Environ. Contam. Toxicol. 25, 95–101 (1993). https://doi.org/10.1007/BF00230718
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00230718