Summary
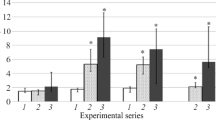
Serum haptoglobin was added to the reaction mixture of prostaglandin synthase (EC 1.14.99.1) and its inhibitory effect was studied. [1-14C]Arachidonic acid was used as substrate and the enzyme activity was estimated by monitoring the radioactivity of the products after thin layer chromatography. With or without addition of hemoglobin to the reaction mixture, both the purified haptoglobin 1-1 and 2-2 showed inhibitory activity. In the presence of 5 μM hematin, however, inhibitory activity of haptoglobin was not observed. Inhibition of prostaglandin synthesis in the system depended on the molar ratio of haptoglobin to hemoglobin in the reaction mixture. These results demonstrate that haptoglobin inhibits prostaglandin synthase by restricting available heme group for the enzyme activity through complexing with hemoglobin. However, haptoglobin did not inhibit completely the stimulatory effect of free hemoglobin. Relevant significance of this effect was discussed.
Similar content being viewed by others
Abbreviations
- Hp:
-
haptoglobin
- EIPS:
-
endogenous inhibitor of prostaglandin synthase
- Hb:
-
hemoglobin
- Hp-Hb:
-
haptoglobin-hemoglobin complex
References
Lands, W. E. M. and Samuelsson, B., 1968. Biochem. Biophys. Acta 164: 426–429.
Bergström, S., Carlson, L. A. and Weeks, J. R., 1968. Pharmacol. Rev. 20: 1–40.
Ramwell, P. W., Shaw, J. E., Clarke, G. B., Grostic, M. F., Keiser, D. G. and Pike, J. E., 1968. Prog. Chem. Fat Other Lipids 9: 233–273.
Hemler, M. and Lands, W. E. M., 1976. J. Biol. Chem. 251: 5575–5579.
Miyamoto, T., Ogino, M., Yamamoto, S. and Hayaishi, O., 1976. J. Biol. Chem. 251: 2629–2636.
Van der Ouderaa, F. J., Buytenhek, M., Nugteren, D. H. and Van Dorp, D. A., 1977. Biochim. Biophys. Acta 487: 315–331.
Ogino, N., Miyamoto, T., Yamamoto, S. and Hayaishi, O., 1977. J. Biol. Chem. 252: 890–895.
Christ-Hazelhof, E. and Nugteren, D. H., 1979. Biochim Biophys. Acta 572: 43–51.
Shimizu, T., Yamamoto, S. and Hayaishi, O., 1979. J. Biol. Chem. 254: 5222–5228.
Roth, G. J., Sick, C. J. and Ozols, J., 1980. J. Biol. Chem. 255: 1301–1304.
Shim, B.-S., 1976. Korean J. Biochem. 8: 7–11.
Saeed, S. A., McDonald-Gibson, W. J., Cuthbert, J., Copas, J. L., Schneider, C., Butt, N. M. and Collier, H. O. J., 1977. Nature (London) 270: 32–36.
Vane, J. R., 1971. Nature (London) New Biol. 231: 232–235.
Kang, Y.-S., Jue, D.-M. and Shim, B.-S., 1979. Korean J. Biochem. 10: 64–65.
Kang, Y.-S., Jue, D.-M. and Shim. B.-S., 1979. Korean J. Biochem. 11: 21–25.
Benesch, R. E., Benesch, R., Renthal, R. D. and Maeda, N., 1972. Biochemistry 11: 3576–3582.
Chu, T. C. and Chu, E. J.-H., 1955. J. Biol. Chem. 212: 1–7.
Wallach, D. P. and Daniels, E. G., 1971. Biochim. Biophys. Acta 231: 445–447.
Han, S.-M. and Shim, B.-S., 1965. Korean Central J. Med. 9: 453–460.
Shim, B.-S., Park, Y.-H., Kim, Y.-J. and Lee, T.-H., 1959. J. Cath. Med. Coll. (Seoul) 3: 176–182.
Cameron, B. F., 1965. Anal. Biochem. 11: 164–169.
Paul, K. G., Theorell, H. and Akeson, A., 1953. Acta Chem. Scand. 7: 1284–1287.
Lowry, O. H., Rosenberg, H. J., Farr, A. L. and Randall, R. J., 1951. J. Biol. Chem. 193: 265–275.
Shim, B.-S. and Bearn, A. G., 1964. J. Exp. Med. 120: 611–628.
Shim, B.-S., Lee, T.-H. and Kang, Y.-S., 1965. Nature(London) 207: 1264–1267.
Shim, B.-S., 1973. Genetics, Structure and Function of Serum Haptoglobin pp. 10–25, Catholic Medical College, Seoul.
Van der Ouderaa, F. J., Buytenhek, M., Slikkerveer, F. J. and Van Dorp, D. A., 1979. Biochim. Biophys. Acta 572: 29–42.
Waks, M., Alfsen, A., Schwaiger, S. and Mayer, A., 1969. Arch. Biochem. Biophys. 132: 268–278.
Hong, K.-J., Kim, S.-K., Choi, J.-M., Cho, B.-K. and Shim, B.-S., 1973. Korean Central J. Med. 25: 205–211.
Deby, C., Van Caneghem, P. and Bacq, Z. M. 1978. Biochem. Pharmacol. 27: 613–615.
Shim, B.-S., 1976. Nature (London) 259: 326–327.
Hong, K.-J. and Shim, B.-S., 1977. Korean J. Biochem. 9: 5–13.
Shim, B.-S. and Kim, I.-K., 1978. Vox Sang. 35: 414–419.
Horrobin, D. F., 1978. Prostaglandins: Physiology, Pharmacology and Clinical Significance, Churchill Livingstone, Edinburgh, Scotland.
Weinberg, J. B. and Hibbs, J. B., Jr., 1977. Nature(London) 269: 245–247.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jue, DM., Shim, BS. & Kang, YS. Inhibition of prostaglandin synthase activity of sheep seminal vesicular gland by human serum haptoglobin. Mol Cell Biochem 51, 141–147 (1983). https://doi.org/10.1007/BF00230400
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00230400