Abstract
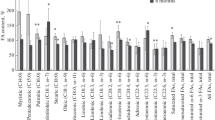
Rats bearing the Yoshida AH-130 ascites hepatoma showed important changes in lipid metabolism. The presence of this rapidly growing tumour induced a significant reduction in the intestinal absorption of an oral [l4C]triolein load but without changes in whole body oxidation of the tracer to CO3. Both white (WAT) and brown (BAT) adipose tissue lipoprotein lipase (LPL) activities were increased at day 4 of tumour growth, changes that seem to be related with those observed in [14C] lipid accumulation; however, heart LPL activity was increased at day 7 but there was no change at day 4. In addition, there was a marked hyperlipemia in the tumour-bearing animals, whereas the blood ketone body concentrations were lower in these animals in comparison with the corresponding pair-fed group. The in vivo lipogenic rate was increased in liver of the tumour-bearing animals (day 4); conversely, it was decreased in WAT and skeletal muscle (day 4) and IBAT (day 7) of the AH-130-bearing rats. It may be suggested that the increased liver lipogenic rate associated with tumour burden is the main factor contributing to the hyperlipidaemia present in the Yoshida AH-130 bearing rats.
Similar content being viewed by others
References
Argilés JM, Azcón-Bieto J: The metabolic environment of cancer. Molec Cell Biochem 81: 3–17, 1988
Argilés JM, López-Soriano FJ: Why do cancer cells have such a high glycolytic rate? Med Hypotheses 32: 151–155, 1990
Medina MA, Sánchez-Jiménez F, Márquez J, Rodríguez-Quesada A, Nuñez de Castro I: Relevance of glutamine metabolism to tumor cell growth. Molec Cell Biochem 113: 1–15, 1992
Axelrod L, Costa G: Contribution of fat loss to weight loss in cancer. Nutr Cancer 2: 81–83, 1980
Watson WS, Sammon AM: Body composition in cachexia resulting from malignant and non-malignant diseases. Cancer 46: 2041–2046, 1980
Costa G, Holland JF: Effects of Krebs-2 carcinoma on the lipid metabolism of male Swiss mice. Cancer Res 22: 1081–1083, 1962
Hansell DT, Davies JWL, Burns HJG, Shenkin A: The oxidation of body fuel stores in cancer patients. Ann Surg 204: 637–642, 1986
Waterhouse C, Kemperman JH: Carbohydrate metabolism in subjects with cancer. Cancer Res 31: 1273–1278, 1971
Costa G, Lyles K, Ulrich L: Effects of human and experimental cancer on conversion of 14C-tripalmitine to 14CO2. Cancer 38: 1259–1265, 1976
Thompson MP, Koons JE, Tan ETH, Grigor MR: Modified lipoprotein activities, rates of lipogenesis, and lipolysis as factors to lipid depletion in C57BL mice bearing the preputial gland tumor, ESR-586. Cancer Res 41: 3228–3232, 1981
Lanza-Jacoby S, Lansey SC, Miller EE, Cleary MP: Sequential changes in the activities of lipoprotein lipase and lipogenic enzymes during tumor growth in rats. Cancer Res 44: 5062–5067, 1984
Evans RD, Argiles JM, Williamson DH: Metabolic effects of tumour necrosis factor-α (cachectin) and interleukin-1. Clin Sci 77: 357–364, 1989
Oller do Nascimiento CM, Williamson DH: Evidence for conservation of dietary lipid in the rat during lactation and the immediate period after removal of the litter. Biochem J 239: 233–236, 1986
Stansbie D, Brownsey RW, Crettaz M, Denton RM: Acute effects in vivo of anti insulin serum on rates of fatty acid synthesis and activities of acetyl coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididyrnal adipose tissue of fed rats. Biochem J 160: 413–416, 1976
Nilsson-Ehle P, Ekman R: Rapid, simple and specific assays for lipoprotein lipase and hepatic lipase. Artery 3: 197–209, 1977
Nilsson-Ehle P, Schotz MC: A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res 17: 536–541, 1976
Agius L, Williamson DH: Lipogenesis in interscapular brown adipose tissue of virgin, pregnant and lactating rats. Biochem J 190: 477–480, 1980
Robinson AM, Girard JR, Williamson DH: Evidence for a role of insulin in the regulation of lipogenesis in lactating rat mammary gland. Biochem J 176: 343–346, 1978
Slein MW: D-glucose determination with hexokinase and glucose-6-phosphate dehydrogenase. In: H.U. Bergmeyer (ed.) Methods of Enzymatic Analysis. Academic Press, New York and London, 1963, pp 117–123
Hohorst HJ, Kreutz FH, Bücher T: On the metabolite content and the metabolite concentration in the liver of the rat. Biochem Z 332: 1846, 1959
Williamson DH, Mellanby J, Krebs HA: Enzymic determination of D(−)β-hydroxybutyric acid and acetoacetic acid in blood. Biochem J 228: 727–733, 1962
Albano JDM, Ekins RP, Maritz G, Turner RC: A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol 70: 487–509, 1972
Eggstein M, Kreutz FH: A new method for the determination of neutral fats in blood serum and tissues. Klin Wochensch 44: 262–267, 1966
Eggstein M, Kuhlmann E: Triglycerides and glycerol. Determination after alkaline hydrolysis. In: H.U. Bergmeyer (ed.) Methods of Enzymatic Analysis. Academic Press, New York, 1974, pp 1825–1831
Shimizu S, Inoue K, Tani Y, Yamada H: Enzymatic microdetermination of serum free fatty acids. Anal Biochem 98: 341–345, 1979
Marzábal M, García-Martínez C, Comas J, López-Soriano FJ, Argilés JM: A flow cytometric study of the rat Yoshida AH-130 ascites hepatoma. Cancer Lett 72: 169–173, 1993
Costelli P, Carbó N, Tessitore L, Bagby GJ, López- Soriano FJ, Argilés JM, Baccino FM: Tumor necrosis factor-α mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 92: 2783–2789, 1993
Mider GB, Sherman CD Jr, Morton JJ: The effects of Walker carcinoma 256 on the total lipid content of rats. Cancer Res 9: 222–229, 1949
Tessitore L, Bonelli G, Baccino FM: Regulation of protein turnover versus growth state: Ascites hepatoma. Arch Biochem Biophys 25: 372–384, 1993
Tessitore L, Bonelli G, Baccino FM: Early development of protein metabolic perturbations in the liver and skeletal muscle of tumor-bearing rats. A model system for cancer cachexia. Biochem J 241: 153–159, 1987
Evans RD, Williamson DH: Tissue-specific effects of rapid tumour growth on lipid metabolism in the rat during lactation and on litter removal. Biochem J 252: 65–72, 1988
Mays T: Cancer and lipid depletion. J Surg Oncol 3: 487–499, 1971
Brenneman DE, Mathur SN, Spector AA: Characterization of the hyperlipidemia in mice bearing the Ehrlich ascites tumor. Eur J Cancer 11: 225–230, 1975
Posner I: Abnormal fat absorption and utilization in rats bearing Walker carcinoma 256. Cancer Res 20: 551–562, 1960
Masuno H, Shiosaka T, Itoh Y, Onji M, Ohta Y, Okuda H: Hepatic triglyceride lipase and lipoprotein lipase activities in post-heparin plasma of patients with various cancers. Jpn J Cancer Res 76: 202–207, 1985
Braun JEA, Severson DL: Regulation of the synthesis, processing and translocation of lipoprotein lipase. Biochem J 287: 337–347, 1992
Mulligan HD, Tisdale MJ: Lipogenesis in tumour and host tissues in mice bearing colonic adenocarcinomas. Br J Cancer 63: 719–722, 1991
Kannan R, Lyon I, Baker N: Dietary control of lipogenesis in vivo in host tissues and tumors of mice bearing Ehrlich ascites carcinoma. Cancer Res 40: 4606–4611, 1980
Noguchi Y, Vydelyngum NA, Conlon KC, Brennan MF: Hypertriglyceridemia in the tumor bearing state is associated with decreased hepatic malic enzyme activity. Surg Forum 39: 462–463, 1988
Argilés JM, García-Martínez C, Llovera M, López-Soriano FJ: The role of cytokines in muscle wasting: Its relation with cancer cachexia. Med Res Rev 6: 637–652, 1992
Tessitore L, Costelli P, Baccino FM: Humoral mediation of cachexia in tumour bearing rats. Br J Cancer 67: 15–23, 1993
Carbo N, Costelli P, Tessitore L, Bagby GJ, López-Soriano FJ, Baccino FM, Argiles JM: Anti-tumour necrosis factor-α treatment interferes with changes in lipid metabolism in a tumour cachexia model. Clin Sci 87: 349–355, 1994
Feingold KR, Serio MK, Adi S, Moser AH, Grunfeld C: Tumour necrosis factor stimulates hepatic lipid synthesis and secretion. Endocrinology 124: 2336–2342, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
López-Soriano, J., Argilés, J.M. & López-Soriano, F.J. Lipid metabolism in rats bearing the Yoshida AH-130 ascites hepatoma. Mol Cell Biochem 165, 17–23 (1996). https://doi.org/10.1007/BF00229741
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229741