Abstract
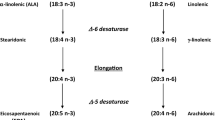
Dietary n-6 and n-3 polyunsaturated fatty acids (PUFAs) have potent biological effects on the blood(cells), the vasculature and the myocardium. In the epidemiological studies in which the benefit from the regular ingestion of n-3 PUFAs was reported, the responsible mechanisms remain obscure. A great deal of the PUFA-effect can be explained by the known interference with the eicosanoid metabolism. Many processes, believed to be involved in atherogenesis such as adhesion and infiltration of bloodcells (in)to the vasculature, platelet aggregation, secretion of endothelium-derived factors and mitogenic responses of vascular smooth muscle cells are partially mediated by receptor-activated phospholipases C-β and A2. As PUFAs take part at many steps of the signalling pathways, the latter could represent important action sites to beneficially interfere with atherogenesis. In this brief review, we have discussed the results of studies on the influence of alteration of PUFA composition of the membrane phospholipids or of exogenously administered non-esterified PUFAs on phospholipid signalling. For convenience, we have mainly focused our discussion on those studies available on the myocardium. By changing the PUFA composition of the phospholipids, the endogenous substrates for the membrane-associated phospholipase C-β and A2 are changed. This is accompanied by changes in their hydrolytic action on these substrates resulting in altered products (the molecular species of 1,2-diacylglycerols and the non-esterified PUFAs) which on their turn evoke changes in events downstream of the signalling cascades: activation of distinct protein kinase C isoenzymes, formation of distinct eicosanoids and non-esterified PUFA effects on Ca 2+ channels. It has also become more clear that the membrane physicochemical properties, in terms of fluidity and cholesterol content of the bilayer, might undergo changes due to altered PUFA incorporation into the membrane phospholipids. The latter effects could have consequences for the receptor functioning, receptor-GTP-binding protein coupling, GTP-binding protein-phospholipase C-β or A2 coupling as well. It should be noted that most of these studies have been carried out with cardiomyocytes isolated from hearts of animals on PUFA diet or incubation of cultured cardiomyocytes with non-esterified PUFAs in the presence of albumin. Studies need to be performed to prove that the PUFA-diet induced modulations of the phospholipid signalling reactions do occur in vivo and that these effects are involved in the mechanism of beneficial effects of dietary PUFAs on the process of atherosclerosis.
Similar content being viewed by others
References
Bang HO, Dyerberg J, Home N: The composition of food consumed by Greenland Eskimos. Acta Med Scand 200: 69–73, 1976
Kagawa Y, Nishizawa M, Suzuki M, Miyatake T, Hamamoto T, Goto K, Motonaga E, Izumikawa H, Hirata H, Ebihara A: Eicosapolyenoic acids of serum lipids of japanese islanders with low incidence of cardiovascular diseases. J Nutr Sci Vitaminol 28: 441–453, 1982
Kromhout D, Bosschieter EB, De Lenzenne Coulander C: The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 312: 1205–1209, 1985
Harris WS: Fish oils and plasma lipid metabolism in humans: a critical review. J Lipid Res 30: 785–807, 1989
Sassen LMA, Lamers JMJ, Verdouw PD: Fish oil and the prevention and regression of atherosclerosis. Cardiovasc Drugs Ther 8: 179–191, 1994
Leaf A: Some effects of ω3 fatty acids on coronary heart disease. In: C. Galli C, A.P. Simopoulos, E. Tremoli, Basel, Karger (eds). Effects of Fatty Acids and Lipids in Health and Disease. World Rev Diet, (eds). vol 76, 1994, pp 1–8
Drevon CA: Marine oils and their effects. Nutrition Rev 50: 38–45, 1992
Lands WEM: Biochemistry and physiology of n-3 fatty acids. FASEB J 6: 2530–2536, 1992
Goodnight SH: Fish oil and vascular disease. Trends Cardiovasc Med 1: 112–116, 1991
Schmidt EB, Kristensen SD, Caterina RD, Illingworth DR: The effects of n-3 fatty acids on plasma lipids and lipoproteins and other cardiovascular risk factors in patients with hyperlipidaemia. Atherosclerosis 103: 107–121, 1993
Soei LK, Lamers JMJ, Sassen LMA, van Tol A, Scheek LM, Dekkers DHW, van Meegen JR, Verdouw PD: Fish oil: A modulator of experimental atherosclerosis in animals. In: S.D. Kristensen, E.B. Schmidt, R. de Caterina, S. Enders (eds). N-3 Fatty Acids: Prevention and Treatment in Vascular Disease. Springer Verlag, London, 1995, pp 55–75
De Jonge HW Van Heugten HAA, Bezstarosti K, Lamers JMJ: Distinct α1-adrenergic agonist and endothelin-l-evoked phosphatidylinositide cycle responses in cultured neonatal rat cardiomyocytes. Bioch Biophys Res Commun 203: 422–429, 1994
Berridge M: Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993
Sluiter W, Pietersma A, Lamers JMJ, Koster JF: Leukocyte adhesion molecules on the vascular endothelium: their role in pathogenesis of cardiovascular disease and the mechanisms underlying their expression. J Cardiovasc Pharmacol 22: S37–44, 1993
Davies PF, Tripathi SC: Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res 72: 239–245, 1993
Rubyani GM: The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol 22: S1–14, 1993
Meldolesi J: Lipid metabolites and growth factor action. TIPS 12: 362–364, 1991
Lamers JMJ, Dekkers DHW, De Jong N, Meij JTA: Modification of fatty acid composition of the phospholipids of cultured rat ventricular myocytes and the rate of phosphatidylinositol-4,5-bisphosphate hydrolysis. J Mol Cell Cardiol 24: 605–618, 1992
Medini L, Colli S, Mosconi E, Tremoli E, Galli C: Diets rich in n-9, n6 and n-3 fatty acids differentially effect the generation of inositol phosphates and of thromboxane by stimulated platelets, in the rabbit. Biochem Pharmacol 39: 129–133, 1990
Skuladottir GV, Schiöth HB, Gudbjamason S: Polyunsaturated fatty acids in heart muscle and α1-adrenoceptor binding properties. Biochim Biophys Acta 1178: 49–54, 1993
Tisdale MJ: Mechanism of lipid mobilization associated with cancer cachexia: interaction between polyunsaturated fatty acid, eicosapentaenoic acid and inhibitory guanine nucleotide-regulatory protein. Prostaglandins Leukotr Essent Fatty Acids 48: 105–109, 1993
May CL, Southworth J, Calder PC: Inhibition of lymphocyte protein kinase C by unsaturated fatty acids. Biochem Biophys Res Commun 195: 823–828, 1993
Brenner RR: Nutritional and hormonal factors including desaturation of essential fatty acids. Prog Lipid Res 20: 41–47, 1982
Tahin QS, Blum M, Carafoli E: The fatty acid composition of subcellular membranes of rat liver, heart, and brain: diet-induced modifications. Eur J Biochem 121: 5–13, 1981
Stubbs CD, Smith AD: The modification of mammalian polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta 779: 89–137, 1984
Lamers JMJ: Dietary eicosapentaenoic acid supplementation can suppress signal transduction at the level of phosphatidyl-inositol-4,5-bisphosphate-specific phospholipase C. Pharm Pharmacol Lett 3: 128–132, 1993
Lamers JMJ, Hartog JM, Verdouw PD, Hulsmann WC: Dietary fatty acids and the myocardial function. Basic Res Cardiol 82: 209–221, 1987
Harris WS: Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res 30: 785–807, 1989
Leaf A: Health claims: omega-3 fatty acids and cardiovascular disease. Nutr Rev 50: 150–154, 1992
Woodcock EA, Anderson KE, Du X-J, Dart AM: Effects of dietary fat supplementation on inositol phosphate release and metabolism in rat left atria. J Mol Cell Cardiol 27: 867–871, 1995
Montfoort A, Van der Werf L, Hartog JM, Hugenholtz PG, Verdouw PD, Hulsmann WC, Lamers JMJ: The influence of fish oil diet and norepinephrine treatment on fatty acid composition of rat heart phospholipids and the positional fatty acid distribution in phosphatidylethanolamine. Basic Res Cardiol 81: 289–302, 1989
Liautaud S, Grynberg A, Mourot J, Athias P: Fatty acids of hearts from rats fed linseed or sunflower oil and of cultured cardiomyocytes grown on their sera. Cardioscience 2: 55–66, 1991
Grynberg A, Fantini E, Athias P, Degois M, Guenot L, Courtois M, Khatami S: Modification of the n-6/n-3 fatty acid ratio in the phospholipids of rat ventricular myocytes in culture by the use of synthetic media: functional and biochemical consequences in normoxic and hypoxic conditions. J Mol Cell Cardiol 20: 863–874, 1988
Grynberg A, Nalbone G, Leonardi J, Lafont H, Athias P: Eicosapentaenoic and docosahexaenoic acids in cultured rat ventricular myocytes and hypoxia induced alterations of phospholipase-A activity. Mol Cell Biochem 116: 75–78, 1992
Kang JX, Leaf A: Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiomyocytes. Proc Natl Acad Sci 91: 9886–9890, 1994
Kang JX, Leaf A: Prevention and termination of β-adrenergic agonist-induced arrhythmias by free polyunsaturated fatty acids in neonatal rat cardiac myocytes. Biochem Biophys Res Commun 208: 629–636, 1995
Hallaq H, Smith TW, Leaf A: Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci 89: 1760–1764, 1992
Bordoni A, Baigi PL, Turchetto E, Rossi CA, Hrelia S: Diacylglycerol fatty acid composition is related to activation of protein kinase C in cultured cardiomyocytes. Cardioscience 3: 251–255, 1992
De Jonge HW Dekkers DHW, Bastiaanse EML, Bezstarosti K, van der Laarse A, Lamers JMJ: Eicosapentaenoic acid incorporation in membrane phospholipids modulates receptor-mediated phospholipase C and membrane fluidity in rat ventricular myocytes. J Mol Cell Cardiol, in press, 1996
Grynberg A, Athias P, Degois M: Effect of change in growth environment on cultured myocardial cells investigated in standard medium. In Vitro Cell Develop Biol 22: 44–50, 1986
Dusserre E, Pulcini T, Bourdillon MC, Ciavatti M, Berthezene F: w-3 Fatty acids in smooth muscle cell phospholipids increase membrane cholesterol efflux. Lipids 30: 35–41, 1995
Nalbone G, Grynberg A, Chevalier A, Leonardi J, Termine E, Lafont H: Phospholipase A activity of cultured rat ventricular myocyte is affected by the nature of cellular polyunsaturated fatty acids. Lipids 25: 301–306, 1990
Kinsella BT, O' Mahony DJ: Lipid modification of G-proteins. Trends Cardiovasc Med 4: 27–34, 1994
Graber R, Sumida C, Nunez EA: Fatty acids and cell signal transduction. J Lipid Mediators Cell Signalling 9: 91–116, 1994
Scherer RW, Breitwieser GE: Arachidonic acid metabolites alter G protein-mediated signal transduction in heart. Effects on muscarinic K+ channels. J Gen Physiol 96: 735–755, 1990
Lochner R, Vogt E, Steiner A, Vetter W: The phosphoinositide turnover of vascular smooth muscle cells is influenced by fish oil. J Hypertension 6 (suppl 4): S222–224, 1988
Lochner R, Schinicks A, Steiner A, Vogt E, Vetter W: Fish oil affects phosphoinositide turnover and thromboxane A metabolism in cultured vascular muscle cells. Biochim Biophys Acta 1012: 279–283, 1989
Rghupathi R, Franson RC: Inhibition of phospholipase A2 by cis-unsaturated fatty acid: evidence for the binding of fatty acid to the enzyme. Biochim Biophys Actal 126: 206–214, 1992
Sadoshima J-I, Izumo S: Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J 12: 1681–1692, 1993
Kriegsmann J, Müller W-D, Richter W, Wunderlich J, Wallukat G: Demonstration of membrane associated phospholipase A2 in cultivated heart muscle cells by immunogold-technique in surface replicas. Acta Histochemica 95: 61–66, 1993
Chen J, Engle SJ, Seilhamer JJ, Tischfield JA: Cloning and recombinant expression of a novel human low molecular weight Ca2+-dependent phospholipase A2. J Biol Chem 269: 2365–2368, 1994
Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL: A novel arachidonic acid-selective cytosolic PLA, contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 65: 1043–1051, 1991
Hazen SL, Hall CR, Ford DA, Gross RW: Isolation of a human myocardial cytosolic phospholipase A2 isoform- Fast atom bombardment mass spectroscopic and reverse-phase high pressure liquid chromatography identification of choline and ethanolamine glycerophospholipid substrates. J Clin Invest 91: 2513–2522, 1993
Vergel R, Mieras MCE, de Haas GH: Action of phospholipase A at interfaces. J Biol Chem 248: 4023–1034, 1972
Sevanian A, Stein RA, Mead JF: Metabolism of epoxidized phosphatidylcholine by phospholipase A2 and epoxide hydrolase. Lipids 16: 781–789, 1981
Mori T, Takai Y, Yu B, Takahashi J, Nishizuka Y, Fujikura T: Specificity of the fatty acyl moieties of diacylglycerol for the activation of calcium-activated, phospholipid-dependent protein kinase. J Biochem 92: 427–431, 1982
Berridge M: Inositol trisphosphate and diacylglycerol as second messengers. Biochem J 220: 345–360, 1984
Anderson KE, Du X-J, Dart AM, Woodcock EA: Effect of dietary n-3 fatty acids on reperfusion-induced release of inositol(1,4,5)-trisphosphate and arrhythmias. In: Proceedings of The Satellite Symposium on: ‘Signal transduction in normal and diseased myocardium’, Rotterdam, The Netherlands, June 30, July 1, 1995
Zhu Y, Nosek TM: Inositol trisphosphate enhances Ca2+-induced Ca2+ release rom cardiac sarcoplasmic reticulum. Pflugers Arch 418: 1–6, 1991
Go M, Sekiguchi K, Nomura H, Kikkawa U, Nishizuka Y: Further studies on the specificity of diacylglycerol for protein kinase C activation. Biochem Biophys Res Commun 144: 598–605, 1987
Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB: Arachidonic acid metabolism. Annu Rev Biochem 55: 69–102, 1986
Lamers JMJ, Dekkers DHW, Mesaeli N, Meij JTA, Panagia V, Van Heugten HAA: Myocardial phosphoinositides do not share the same fatty acid profile. Biochem Biophys Res Common 191: 487–494, 1993
Damron DS, Bond M: Modulation of Ca2+ cycling in cardiac myocytes by arachidonic acid. Circ Res 72: 376–386, 1993
Carafoli E: The signalling function of calcium and its regulation. J Hypertension 12: S47–56, 1994
Barry WH, Bridge JHB: Intracellular calcium homeostasis in cardiac myocytes. Circ 87: 1806–1815, 1993
Van Heugten HAA, De Jonge HW, Bezstarosti K, Lamers JMJ: Calcium and the endothelin-1 and α1-adrenergic stimulated phosphatidylinositol cycle in cultured rat cardiomyocytes. J Mol Cell Cardiol 26: 1081–1093, 1994
Hallaq H, Sellmayer A, Smith TW, Leaf A: Protective effect of eicosapentaenoic acid on ouabain toxicity in neonatal rat cardiac myocytes. Proc Natl Acad Sci 87: 7834–7838, 1990
Pepe S, Bogdanov K, Hallaq H, Spurgeon H, Leaf A, Lakatta E: ω-3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2−, and contraction in adult rat cardiac myocytes. Proc Natl Acad Sci 91: 8832–8836, 1994
Huang JM-C, Xian H, Bacaner M: Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci 89: 6452–6456, 1992
Lamers JMJ, Stinis JT, Montfoort A, Hülsmann WC: The effect of lipid intermediates on Ca2+ and Na+ permeability and (Na++K+)-ATPase of cardiac sarcolemma. Biochim Biophys Acta 774: 127–137, 1984
Taffet GE, Pham TT, Bick DLM, Entman ML, Pownall HJ, Bick RJ: The calcium uptake of the rat heart sarcoplasmic reticulum is altered by dietary lipid. J Membrane Biol 131: 35–42, 1993
McPhail LC, Clayton CC, Snyderman R: A potential second messenger role for poly unsaturated fatty acids of Ca 2+-dependent protein kinase. Science 224: 622–625, 1984
Nishizuka, Y: Intracellular signalling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992
Steinberg SF, Goldberg M, Rybin VO: Protein kinase C isoform diversity in the heart. J Mol Cell Cardiol 27: 141–153, 1995
Nakamura S, Asaoka Y, Yoshida K, Sasaki Y, Nishizuka Y: Protein kinase C for cell signalling: a possible link between phospholipases. In: B.L. Brown, P.R.M. Dobson (eds). Advances in Second Messenger and Phosphoprotein Research. 28: 171–178, 1993
Lamers JMJ, Eskildsen-Helmond YEG, Resink AM, de Jonge HW, Bezstarosti K, Sharma HS, van Heugten HAA: Endothelin-1 induced phospholipase C-β and D and protein kinase C-isoenzyme signalling leading to hypertrophy in rat cardiomyocytes. J Cardiovasc Pharmacol, 26, S100–103, 1995
Eskildsen-Helmond YEG, Van Heugten HAA, Lamers JMJ: Regulation and functional significance of phospholipase D in myocardium. Mol Cell Biochim 157: 39–40, 1996 (this volume)
De Jonge HW Van Heugten HAA, Lamers JMJ: Review: Signal transduction by the phosphatidylinositol cycle in myocardium. J Mol Cell Cardiol 27: 29–106, 1995
Bell JG, Dick JR, McVicar AH, Sargent JR, Thompson KD: Dietary sunflower, linseed and fish oils affect phospholipid fatty acid composition, development of cardiac lesions, phospholipase activity and eicosanoid production in atlantic salmon (Salmo salar). Prostaglandins Leukotr Essent Fatty Acids 49: 665–673, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Jonge, H.W., Dekkers, D.H.W. & Lamers, J.M.J. Polyunsaturated fatty acids and signalling via phospholipase C-β and A2 in myocardium. Mol Cell Biochem 157, 199–210 (1996). https://doi.org/10.1007/BF00227899
Issue Date:
DOI: https://doi.org/10.1007/BF00227899