Summary
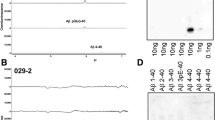
A monoclonal antibody (AmT-1) produced against synthetic amyloid β peptide (1–28 residues) was revealed to be reactive with amyloid β peptide blotted on nitrocellulose membrane, but not with that dissolved in sodium dodecyl sulfate and electrophoresed. AmT-1 immunostained senile plaques of typical, primitive and diffuse type, as well as amyloid deposits in cerebral vessels. It also reacted with neuronal and glial cells of normal and Alzheimer's disease (AD) brains. In addition, AmT-1 was also reactive strongly with lipofuscin pigments of adrenal reticular cells, and weakly with those of eccrine glands and liver cells. A rat neural cell line (PC12h) was reactive with AmT-1. By immunoelectron microscopy, a positive reaction was seen in ribosomes along the rough endoplasmic reticulum of nerve cells and PC12h cells. By immunoprecipitation, AmT1 reacted with a band at 36 kDa in the brain homogenates from Ad patients as well as from normal aged subjects. By immunoblotting analysis, AmT1 reacted with a band at 36 kDa in the cytosolic fraction of PC12 cells, and three bands (12–17 kDa) in the lipopigment fraction of the adrenal gland. These findings suggest that the cerebral amyloid deposits contain substance(s) having an epitope common to neuronal cells and lipofuscin pigments. The possible relationship between cerebral amyloid deposits and lipofuscin pigments in systemic organs is discussed.
Similar content being viewed by others
References
Allsop D, Wong CW, Ikeda A, Landon M, Kidd M, Glenner GG (1988) Immunohistochemical evidence for the derivation of a peptide ligand from the amyloid β-protein precursor of Alzheimer disease. Proc Natl Acad Sci USA 85:2790–2794
Bahmanyar S, Higgins GA, Goldgaber D, Lewis DA, Morrison JH, Wilson MC, Shankar SK, Gajdusek DC (1987) Localization of amyloid β protein mRNA in brains from patients with Alzheimer's disease. Science 237:77–80
Bancher C, Grundke-Iqbal I, Iqbal K, Kim KS, Wisniewski HM (1989) Immunoreactivity of neuronal lipofuscin with monoclonal antibodies to the amyloid β-protein. Neurobiol Aging 10:125–132
Benowitz LI, Rodrigues W, Paskevich P Mufson EJ, Scenk D, Neve RL (1989) The amyloid precursor protein is concentrated in neuronal lysosomes in normal and Alzheimer disease subjects. Exp Neurol 106:237–250
Card JP, Maede RP, Davis LG (1988) Immunocytochemical localization of the precursor protein for β-amyloid in the rat central nervous system. Neuron 1:835–846
Catteruccia N, Willingale-Theune J, Bunke D, Prior R, Masters CL, Crisanti A, Beyreuther K (1990) Ultrastructural localization of the putative precursors of the A4 amyloid protein associated with Alzheimer's disease. Am J Pathol 137:19–26
Cohen ML, Golde TE, Usiak MF, Youkin LH, Youkin SG (1988) In situ hybridization of nucleus basalis neurons shows increased β amyloid mRNA in Alzheimer's disease. Proc Natl Acad Sci USA 85:1227–1231
Cras P, Kawai M, Siedlak S, Mulvihill P, Gambetti P, Lowery D, Gonza'lez-Dewhitt P, Greenberg B, Perry G (1990) Neuronal and microglial involvement in β amyloid protein deposition in Alzheimer's disease. Am J Pathol 137:241–246
Glenner GG, Wong CW (1984) Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Glenner GG, Wong CW (1984) Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122:1131–1135
Golde TE, Estus S, Usiak M, Younkin LH, Younkin SG (1990) Expression of β amyloid protein precursor mRNA: recognition of a novel alternatively spliced form and quantification in Alzheimer's disease using PCR. Neuron 4:253–267
Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC (1987) Characterization and chromosomal localization of a DNA encoding brain amyloid of Alzheimer's disease. Science 235:877–880
Grunke-Iqbal I, Iqbal K, Tung Y-C, Kim KS, Wisniewski HM (1989) Amyloid protein and neurofibrillary tangles coexist in the same neuron in Alzheimer disease. Proc Natl Acad Sci USA 86:2853–2857
Hatanaka H (1981) Nerve growth factor-mediated stimulation of tyrosine hydroxylase activity in a clonal rat pheochromocytoma cell line. Brain Res 222:225–233
Hirokawa K, Utsuyama M, Morrizumi E, Handa S (1986) Analysis of the thymic microenvironment by monoclonal antibodies with special reference to thymic nurse cells. Thymus 8:349–360
Ikeda S, Allsop D, Glenner GG (1989) The morphology and distribution of plaque and related deposits in the brains of Alzheimer's disease and control cases: an immunohistochemical study using amyloid β-protein antibody. Lab Invest 60:113–122
Joachim CL, Mori H, Selkoe DJ (1989) Amyloid β-protein deposition in tissues other than Alzheimer's disease. Nature 341:226–230
Kang J, Lemaire H-G, Untelbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B, (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325:733–735
Kawasaki H, Utsuyama H, Takahashi H, Hayashi Y, Kurashima C, Esaki Y, Maruyama M, Hirokawa K (1989) Establishment of a monoclonal antibody against senile plaques and its application for immunohistological and immunoelectron microscopical studies in the brain of the elderly. Acta Neuropathol 79:44–47
Kitaguchi T, Wisniewski KE, Maslinski S, Maslinska D, Wisniewski TM, Kim KS (1990) β-protein immunoreactivity in brains of patients with neuronal ceroid lipofuscinosis: ultrastructural and biochemical demonstration. Neurosci Lett 112:155–160
Lewis DA, Higgins GA, Young WG, Goldgaber D, Gadjusek DC, Wilson MC, Morrison JH (1988) Distribution of precursor amyloid β protein messenger RNA in human cerebral cortex: relationship to neurofibrillary tangles and neuritic plaques. Proc Natl Acad Sci USA 85:1691–1695
Mita S, Schon EA, Herbert J (1989) Widespread expression of amyloid β protein precursor gene in rat brain. Am J Pathol 134:1253–1261
Masters CL, Multhaup G, Simms G, Pottigiesser J, Martins RN, Beyreuther K (1985) Neuronal origin of a cerebral amyloid: neurofibrillary tanges of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J 4:2757–2763
Masters CL, Simms G, Weinmann NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer's disease and Down's syndrome. Proc Natl Acad Sci USA 82:4245–4249
Ogomori K, Kitamoto J, Teteishi Y, Sato M, SueTsugu M, Abe M (1989) β-protein amyloid is widely distributed in the central nervous system of patients with Alzheimer's disease. Am J Pathol 134:243–250
Palmer DN, Barns G, Husbands DR, Jolly RD (1986) Ceroid lipofuscinosis in sheep. II. The major component of the lipopigment in liver, kidney, pancreas and brain is low molecular weight protein. J Biol Chem 261:1773–1777
Palmert MR, Berman-Podlisny M, Whitker DS, Oltersdorf Y, Youkin LH, Selkoe DJ, Youkins SG (1988) Antisera to an amino-terminal peptide detect the amyloid protein precursor of Alzheimer's disease and recognize senile plaques. Biochem Biophys Res Commun 156:432–437
Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM (1987) Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci USA 84:4190–4194
Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, Oltersdorf T (1988) β amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kDa membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci USA 85:7341–7345
Shivers BD, Hilbich C, Multhaup G, Salbaum M, Beyreuther, Seeburg PH (1988) Alzheimer's disease amyloidgenic glyco-protein: expression pattern in rat brain suggests a role in cell contact. EMBO J 7:1365–1370
Siman R, Card P, Nelson RB, Davis LG (1989) Expression of β-amyloid precursor protein in reactive astrocytes following neuronal damage. Neuron 3:275–285
Stern RA, Otvos L, Trojanowski JQ, Lee VM-Y (1989) Monoclonal antibodies to a synthetic peptide homologous with the first 28 amino acids of Alzheimer's disease β-protein recognize amyloid and diverse glial and neuronal cell types in the central nervous system. Am J Pathol 135:973–978
Takahashi H, Kurashima C, Utsuyama M, Hirokawa K (1990) Immunohistological study of senile brains by using a monoclonal antibody recognizing β amyloid precursor protein: significance of granular deposits in relation with senile plaques. Acta Neuropathol 80:260–265
Tanzi RE, Gusella JF, Watkins PC, Bruns GAP, St George-Hyslop P, Vankeuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL (1987) Amyloid β protein gene: cDNa, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235:880–883
Tate-Ostroff B, Majocha RE, Marota CA (1989) Identification of cellular and extracellular sites of amyloid precursor protein extracytoplasmic domain in normal and Alzheimer disease brains. Proc Natl Acad Sci USA 86:745–749
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Wisniewski KE, Kida E, Gordon-Majszak W, Saitoh T (1990) Altered amyloid β-protein precursor processing in brains of patients with neuronal ceroid lipofuscinosis. Neurosci Lett 120:94–96
Wisniewski KE, Maslinska D, Kitaguchi T, Kim KS, Goebel HH, Haltia M (1990) Topographic heterogeneity of amyloid β-protein epitopes in brains with various forms of neuronal ceroid lipofuscinoses suggesting defective processing of amyloid precursor protein. Acta Neuropathol 80:26–34
Yamaguchi H, Hirai S, Morimatsu M, Shoji M, Ihara Y (1988) A variety of cerebral amyloid deposits in the brains of the Alzheimer-type dementia demonstrated by β protein immunostaining. Acta Neuropathol 76:541–549
Yamaguchi H, Hirai S, Morimatsu M, Shoji M, Harigaya Y (1988) Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol 77:113–119
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takahashi, H., Utsuyama, M., Kurashima, C. et al. Monoclonal antibody to β peptide, recognizing amyloid deposits, neuronal cells and lipofuscin pigments in systemic organs. Acta Neuropathol 85, 159–166 (1993). https://doi.org/10.1007/BF00227763
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227763