Summary
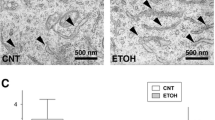
The effects of chronic ethanol exposure on number and calibres of optic nerve axons (and number of retinal ganglion cells) were investigated in a rat model. Male Sprague-Dawley rats were fed a liquid, ethanol-containing diet for 5, 10 and 17 weeks with littermates given isocaloric amounts of ethanol-free diet serving as controls. After fixation by perfusion, the optic nerves were imbedded in epoxy resin and sectioned for electron microscopy. Systematic random sampling was made from a cross-shaped area over the nerve. Axons within a counting frame were counted and morphometrically categorized with regard to mean diameter and the total number of axons estimated from number per area and the cross-sectional area of the nerve, which was measured using a digitizer table. According to non-parametric statistical analysis, ethanol exposure resulted in a significant reduction in mean cross-sectional area of the optic nerve and in mean axonal calibre but not in total axonal number in the ethanol-treated rats but there was no significant effect of duration of the exposure. The mean cross-sectional area of the nerve was reduced by 9%, 10% and 18% after 5, 10 and 17 weeks of exposure, respectively. The reduction in cross-sectional area appeared to be related to a proportional reduction in axonal and myelin area fractions. The findings indicate that chronic ethanol exposure results in decreased axonal calibres without axonal loss. This also implies that there is no reduction in the number of retinal ganglion cells.
Similar content being viewed by others
References
Begleiter H, Porjesz B, Chou CL (1981) Auditory brain stem potentials in chronic alcoholics. Science 211:1064–1066
Béracochéa D, Lescaudron L, Verna A, Jaffard R (1987) Neuroanatomical effect of chronic ethanol consumption on dorsomedial and anterior thalamic nuclei and substantia innominata in mice. Neurosci Lett 73:81–84
Bergman H, Borg S, Hindmarsh T, Ideström C-M, Mutzell S (1980) Computed tomography of the brain and neuropsychological assessment of alcoholic patients. In: Begleiter H (ed) Biological effects of alcohol. Plenum Publishing Corporation, New York, pp 771–786
Cadete-Leite A, Alves MC, Taveres MA, Paula-Barbosa MM (1990) Effects of chronic alcohol intake and withdrawal on the prefrontal neurons and synapses. Alcohol 7:145–152
Cala AL (1985) CT Demonstration of the early effects of alcohol on the brain. In: Galanter M (ed) Recent developments in alcoholism. Plenum Press, New York, pp 253–264
Carlen PL, Wilkinson DA, Wortzman G, Holgate R, Cordingley J, Lee MA, Huszar L, Moddel G, Singh R, Kiraly L, Rankin JG (1981) Cerebral atrophy and functional deficits in alcoholics without clinically apparent liver disease. Neurology 31:377–385
Chu N-S, Squires KC, Starr AA (1982) Auditory brain stem responses in chronic alcoholic patients. Electroencephalogr Clin Neurophysiol 54:418–425
Conradi NG, Rydenhag B, Friberg PA (1986) Connecting a conventional particle size analyser to a micro-computer: a cheap and simple modification giving automatic data handling. Acta Stereol 5:241–243
Conradi NG, Sjöström A, Rydenhag B (1989) Functional development of the visual system in normal and protein-deprived rats. VIII. Post-natal development of optic nerve axons. Acta Physiol Scand 136:597–603
DePergola G, Kjellström C, Holm C, Conradi N, Pettersson P, Björntorp P (1991) The metabolism of ethyl esters of fatty acids in adipose tissue of rats chronically exposed to ethanol. Alcohol Clin Exp Res 15:184–189
Emmerson RY, Dustman RE, Shearer DE, Chamberlain HM (1987) EEG, visually evoked and event related potentials in young abstinent alcoholics. Alcohol 4:241–248
Erwin CW, Linnoila M (1981) Effect of ethyl alcohol on visual evoked potentials. Alcohol Clin Exp Res 5:49–55
Fox JH, Ramsey RG, Huckman MS, Proske AE (1976) Cerebral ventricular enlargement: chronic alcoholics examined by computerized tomography. J Am Med Assoc 236:365–368
Franck H, Gerhard JP, Schalk P, Lanzrath MA, Bronnet A (1985) Les potentiels évoqués visuels peuvent-ils avoir une valeur pronostique chez l'éthylique? Bull Mem Soc Fran Ophthal 96:462–465
Haan J, Lappe-Ostege B, Kordt G (1983) Vizuell evozierte Potentiale bei Alkoholismus. Nervenarzt 54:491–493
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Ishii T (1983) A comparison of cerebral atrophy in CT scan findings among alcoholic groups. Acta Psychiatr Scand 68 [Suppl 309]:1–30
Jensen OL, Krogh E (1984) Visual evoked response and alcohol intoxication. Acta Ophthalmol 62:651–657
Kelley JT, Pena Y, Reilly EL, Overall JE, Colton GS (1984) Effects of age and alcohol abuse on pattern reversal visual evoked potentials. Clin Electroencephalogr 15:102–109
King MA, Hunter BE, Walker DW (1988) Alterations and recovery of dendritic spine density in rat hippocampus following long-term ethanol ingestion. Brain Res 459:381–385
Kjellström C, Almström S, Conradi N (1990) Decrased synapse-to-neuron ratio in rat locus coeruleus following chronic ethanol feeding. Xth International Congress on Neuropathology, Sept 2–8, Kyoto
Kjellström C, Rydenhag B, Sjöström A, Conradi NG (1992). Alterations in cortical evoked response following ethanol feeding in adult rats (submitted)
Lam K, Sefton AJ, Bennett MR (1982) Loss of axons from the optic nerve of the rat during early postnatal development. Dev Brain Res 3:487–491
Lee K, Dunwiddie T, Deitrich R, Lynch G, Hoffer B (1981) Chronic ethanol consumption and hippocampal neuron dendritic spines: a morphometric and physiological analysis. Exp Neurol 71:541–549
Lehmann EL (1975) Nonparametrics, statistical methods based on ranks. Holden-Day, San Fransisco
Lescaudron L, Verna A (1986) Degeneration and regeneration of dendritic spines after chronic alcoholism and withdrawal: a quantitative study in the mouse hippocampus. Neurosci Lett [Suppl] 26:S293
Lescaudron L, Béracochéa D, Verna A, Jaffard R (1984) Chronic ethanol consumption induces neuronal loss in mamillary bodies of the mouse: a quantitative analysis. Neurosci Lett 50:151–155
Lescaudron L, Jaffard R, Verna A (1989) Modifications in number and morphology of dendritic spines resulting from chronic ethanol consumption and withdrawal: a Golgi study in the mouse anterior and posterior hippocampus. Exp Neurol 106:156–163
Lieber CS, DeCarli LM (1982) The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res 6:523–531
Lieber CS, DeCarli LM (1986) The feeding of ethanol in liquid diets. Alcohol Clin Exp Res 10:550–553
Lishman WA (1981) Cerebral disorders in alcoholism. Syndromes of impairment. Brain 104:1–20
McMullen PA, Saint-Cyr JA, Carlen PL (1984) Morphological alterations in rat CA1 hippocampal pyramidal cell dendrites resulting from chronic ethanol consumption and withdrawal. J Comp Neurol 225:111–118
Perry VH, Henderson Z, Linden R (1983) Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol 219:356–368
Phillips SC, Cragg BG (1983) Chronic consumption of alcohol by adult mice: effect on hippocampal cells and synapses. Exp Neurol 80:218–226
Potts RA, Dreher B, Bennett MR (1982) The loss of ganglion cells in the developing retina of the rat. Dev Brain Res 3:481–486
Riley JN, Walker DW (1978) Morphological alterations in hippocampus after long-term alcohol consumption in mice. Science 201:646–648
Shaw S, Gorkin BD, Lieber CS (1981) Effects of chronic alcohol feeding on thiamine status: biochemical and neurological correlates. Am J Clin Nutr 34:856–860
Tabakoff B, Noble EP, Warren KR (1979) Alcohol nutrition and the brain. In: Wurtman RJ, Wurtman JJ (eds) Nutrition and the brain. Raven Press, New York, pp 159–213
Tavares MA, Paula-Barbosa MM (1982) Alcohol-induced granule cell loss in the cerebellar cortex of the adult rat. Exp Neurol 78:574–582
Tavares MA, Paula-Barbosa MM, Gray EG (1983) A morphometric Golgi analysis of the Purkinje cell dendritic tree after long-term alcohol consumption in the adult rat. J Neurocytol 12:939–948
Torvik A, Lindboe CF, Rogde S (1982) Brain lesions in alcoholics. A neuropathological study with clinical correlations. J Neurol Sci 5:233–248
Victor M (1984) Polyneuropathy due to nutritional deficiency and alcoholism. In: Dyck PJ, Thomas PK, Lambert EH, Bunge R (eds) Peripheral neuropathy. W. B. Saunders, Philadelphia, pp 1899–1940
Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P (1980) Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science 209:711–713
Walker DW, Hunter BE, Abraham WC (1981) Neuroanatomical and functional deficits subsequent to chronic ethanol administration in animals. Alcohol Clin Exp Res 5:267–282
Author information
Authors and Affiliations
Additional information
Supported by the Bank of Sweden Tercentenary Foundation (Proj. No. 86/80), the Swedish Medical Research Council (Proj. No. 07121) and Swedish Alcohol Monopoly Foundation for Alcohol Research (89/11)
Rights and permissions
About this article
Cite this article
Kjellström, C., Conradi, N.G. Decreased axonal calibres without axonal loss in optic nerve following chronic alcohol feeding in adult rats: a morphometric study. Acta Neuropathol 85, 117–121 (1993). https://doi.org/10.1007/BF00227757
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227757