Abstract
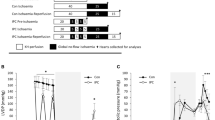
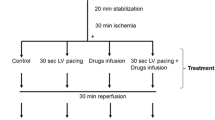
In this study the mass of polyphosphoinositides as well as the turnover of [3H]inositol phospholipids and [3H]inositol phosphates during ischaemia and short periods of reperfusion were studied in the isolated perfused rat heart. Since the phosphoinositides located within the sarcolemma are precursors for release of inositoltrisphosphate (InsP3) and diacylglycerol, sarcolemmal membranes (rather than whole tissue) isolated at the end of the experimental procedure, were used. Hearts were prelabelled with [3H]inositol and subsequently perfused with 10 mM LiCI to block the phosphatidylinositol (PI) pathway. The results showed that 20 min of global ischaemia depressed the amount of [3H]inositol present in both sarcolemmal phosphatidylinositol-4-phosphate (PI-4-P) and phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2), as well as in the cytosolic [3H]inositol phosphates, [3H]InsP2 and [3H]InsP3. The mass of the sarcolemmal inositol phospholipids remained unchanged during ischaemia. Reperfusion caused an immediate (within 30 sec) increase in the amount of [3H]inositol in sarcolemmal PI, PI-4-P and PI-4,5-P2. PI-4-P levels showed a transient increase after 30 seconds postischaemic reperfusion, while the mass of the other sarcolemmal inositol phospholipids, PI and PI-4,5-P2, remained unchanged. [3H]Insp, [3H]InsP2 and [3H]InsP3 also increased significantly in comparison to ischaemic hearts after only 30 sec postischaemic reperfusion.
In summary, the results obtained indicate inhibition of the PI pathway during ischaemia with an immediate significant stimulation upon reperfusion. In view of the capacity of InsP3 to mobilize Ca2+ the possibility exists that stimulation of this pathway during reperfusion may play a role in the intracellular Ca2+ overload, characteristic of postischaemic reperfusion.
Similar content being viewed by others
References
Berridge MJ, Irvine RF: Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312: 315–321, 1984
Berridge MJ, Irvine RF: Inositol phosphates and cell signalling. Nature 341: 197–204, 1989
Nosek TM, Williams MF, Zeigler ST, Godt RE: Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol 250: C807-C811, 1986
Movsesian MA, Thomas AP, Selak M, Williamson JR: Inositol trisphosphate does not release Ca2+ from permeabilized cardiac myocytes and sarcoplasmic reticulum. FEBS Letters 185: 328–332, 1985
Hirata M, Suematsu E, Hashimoto T et al.: Release of Ca2 from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J 223: 229–236, 1984
Mouton R, Lochner A: Inositol phosphates and calcium release from sarcoplasmic reticulum of cardiac muscle. SAJ Science: in press, 1990
Shen AC, Jennings RB: Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol 67: 417–440, 1972
Nayler WG: The role of calcium in the ischemic myocardium. Am J Pathol 102: 262–270, 1981
Philipson KD, Bers DM, Nishimoto AY: The role of phospholipids in the Ca2+ binding of isolated cardiac sarcolemma. J Mol Cell Cardiol 12: 1159–1173, 1980
Lochner A, Sanan D, Victor T et al.: Mitochondrial and sarcolemmal function and integrity in the ischaemic myocardium. In: MC Berman, W Gevers, LH Opie (eds.) Membranes and Muscle. IRL Press, 1985, pp 309–325
Lowry AO, Rosenbrough NJ, Farr AL et al.: Protein with the folin phenol reagent. J Biol Chem 193: 265–275, 1951
Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Physiol 37: 911–917, 1959
Hauser G, Eichberg J: Improved conditions for the preservation and extraction of polyphosphoinositides. Biochim Biophys Acta 326: 201–209, 1973
Shaikh NA, Palmer FBStC: Phosphoinositide kinases in chick brain and sciatic nerve, a developmental study. J Neurochem 28: 395–402, 1977
Itaya K, Ui M: A new micromethod for the colorimetric determination of inorganic phosphates. Clin Chim Acta 14: 361–366, 1966
Woodcock EA, Smith AI, Wallace CA et al.: An unusual phosphatidylinositol turnover pathway in noradrenalineperfused rat hearts. Clin Exp Pharmacol 15: 251–255, 1988
Berridge MJ, Dawson RMC, Downes PC et al.: Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J 212: 473–482, 1983
Irvine RF, Anggard EE, Letcher AJ et al.: Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J 229: 505–511, 1985
Woodcock EA, Schmauk White LB, Smith AI et al.: Stimulation of phosphatidylinositol metabolism in the isolated, perfused rat heart. Circ Res 61: 625–631, 1987
Otani H, Prasad MR, Engelman RM, Otani H, Cordic GA, Das DK: Enchanced phosphodiesteratic breakdown and turnover of phosphoinositides during reperfusion of ischemic rat heart. Circ Res 63: 930–936, 1988
Brown JH, Buxton IL, Brunton LL: α1-Adrenergic and muscarinic cholinergic stimulation of phosphoinositide hydrolysis in adult rat cardiomyocytes. Circ Res 57: 532–537, 1985
Guse AH, Berg I, Gercken G: Metabolism of inositol phosphates in α1-adrenoceptor-stimulated and homogenized cardiac myocytes in adult rats. Biochem 1261: 89–92, 1989
Renard D, Poggioli J: Does the inositol trisltetrakisphosphate pathway exist in rat heart? FEBS Letters 217: 117–123, 1987
Kohl C, Schmitz W, Sholz H et al.: Evidence for the existence of inositol tetrakisphosphate in mammalian heart. Effect of α1-adrenoceptor stimulation. Circ Res 66: 580–583, 1990
Quist E, Satumtira N, Powell P: Regulation of polyphosphoinositide synthesis in cardiac muscle. Arch Biochem Biophys 271: 21–32, 1989
Tibbits GF, Sasaki M, Ikeda M, Shimada K, Tsuruhara T, Takafumi N: Characterization of rat myocardial sarcolemma. J Mol Cell Cardiol 13: 1051–1061, 1981
Schwertz DW, Halverson J, Isaacson T et al.: Alterations in phospholipid metabolism in the globally ischemic rat heart: emphasis on phosphoinositide specific phospholipase C activity. J Mol Cell Cardiol 19: 685–697, 1987
Mouton, Huisamen B, Lochner A: Increased myocardial inositoltrisphosphate levels during α1-adrenergic stimulation and reperfusion of ischaemic rat heart. J Mol Cell Cardiol: accepted for publication, 1990
Kasinathan C, Xu ZC, Kirchberger MA: Polyphosphoinositide formation in isolated cardiac plasma membranes. Lipids 24: 818–823, 1989
Chien KR, Reeves JP, Buja LM et al.: Phospholipid alterations in canine ischemic myocardium. Circ Res 48: 711–719, 1981
Hokin LE: Receptors and phosphoinositide generated second messengers. Ann Rev Biochem 54: 205–235, 1985
Helmkamp GM, Harvey MS, Wirtz KWA et al.: Phospholipid exchange between membranes. J Biol Chem 249: 6382–6389, 1974
Yoshimura T, Helmkamp GM: Bovine brain phosphatidylinositol transfer protein. Effects of pH, ionic strength and lipid composition on transfer activity. Biochim Biophys Acta 793: 463–470, 1984
Helmkamp GM: Effects of phospholipid fatty acid composition and membrane fluidity on the activity of bovine brain phospholipid exchange protein. Biochemistry 19: 2050–2055, 1980
Edoute Y, van der Merwe E, Sanan D, Kotzé JCN, Steinmann C, Lochner A: Normothermic ischaemic cardiac arrest of the isolated working rat heart. Effects of time and reperfusion on myocardial ultrastructure, mitochondrial oxidative function, and mechanical recovery. Circ Res 53: 663–768, 1983
Corr PB, Shayman JA, Kramer JB: Increased α-adrenergic receptors in ischaemic cat myocardium. J Clin Invest 67: 1232–1236, 1981
Movsesian MA, Nishikawa M, Adelstein RS: Phosphorylation of phospholamban by calcium-activated, phospholipid-dependent protein kinase. J Biol Chem 259: 8029–8032, 1984
Lindemann JP: α-Adrenergic stimulation of sarcolemmal protein phosphorylation and slow responses in intact myocardium. J Biol Chem 261: 4860–4867, 1986
Otani H, Otani H, Das DK: α1-Adrenoceptor-mediated phosphoinositide breakdown and inotropic response in rat left ventricular papillary muscles. Circ Res 62: 8–17, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mouton, R., Huisamen, B. & Lochner, A. The effect of ischaemia and reperfusion on sarcolemmal inositol phospholipid and cytosolic inositol phosphate metabolism in the isolated perfused rat heart. Mol Cell Biochem 105, 127–135 (1991). https://doi.org/10.1007/BF00227752
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227752