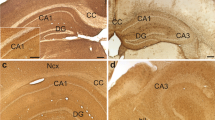
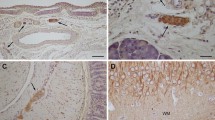
Summary
Surgical specimens from 36 medulloblastomas (25 classic and 11 desmoplastic) were studied by peroxidase-antiperoxidase (PAP) immunohistochemistry with antibodies against the class III β-tubulin isotype (β-tubulin), synaptophysin, retinal S-antigen (S−Ag), and glial fibrillary acidic protein (GFAP). We found that neoplastic cells expressed β-tubulin in 91% of the tumors (23 classic and 10 desmoplastic), synaptophysin in 75% (19 classic and 8 desmoplastic), S−Ag in 44% (11 classic and 5 desmoplastic), and GFAP in 11% of medulloblastomas (2 classic and 2 desmoplastic). Synaptophysin and β-tubulin positivities were observed in undifferentiated neoplastic cells, in cells forming neuroplastic rosettes, and in pale islands, while S−Ag immunopositivity was noted in undifferentiated cells, occasionally in β-tubulin-negative neuroblastic rosettes, and exceptionally in pale islands. Large pale islands, in two desmoplastic medulloblastomas, exhibited distinct patterns of immunoreactivity to the above markers, suggesting neuronal and glial differentiation in the central area, and intense neuritic development in the peripheral zone. Our findings confirm the predominant capacity of medulloblastoma cells to differentiate along neuronal cell lines and indicate that large pale islands, in desmoplastic medulloblastomas, represent well-organized areas for neuronal and, to a lesser degree, astroglial differentiation. Furthermore, it appears, in our cases, that immunohistochemical features do not represent clear-cut prognostic indicators in patients with medulloblastomas.
Similar content being viewed by others
References
Bonnin JM, Perentes E (1988) Retinal S-antigen immunoreactivity in medulloblastomas. Acta Neuropathol 76:204–207
Burger PC, Grahmann FC, Bliestle A, Kleihues P (1987) Differentiation in the medulloblastoma. A histological and immunohistochemical study. Acta Neuropathol (Berl) 73:115–123
Burgoyne RD (1986) Microtubule proteins in neuronal differtentiation. Comp Biochem Physiol 83B:1–8
Caputy AJ, McCullough DC, Manz HJ, Patterson K, Hammock MK (1987) A review of the factors influencing the prognosis of medulloblastoma. J Neurosurg 66:80–87
Coffin CM, Braun JT, Wick MR, Dehner LP (1990) A clinicopathologic and immunohistochemical analysis of 53 cases of medulloblastoma with emphasis of synaptophysin expression. Mod Pathol 3:164–170
Cruz-Sanchez FF, Rossi ML, Hughes JT, Esiri MM, Coakham HB (1989) Medulloblastoma. An immunohistological study of 50 cases. Acta Neuropathol 79:205–210
Cudkowicz M, De la Monte SM (1989) Histogenesis and cell lineage analysis of medulloblastomas. J Neurol Sci 94:221–229
Czerwionka M, Korf HW, Hoffmann O, Busch H, Schachenmayr W (1989) Differentiation in medulloblastomas: correlation between the immunocytochemical demonstration of photoreceptor markers (S-antigen, rod-opsin) and the survival rate in 66 patients. Acta Neuropathol 78:629–636
Donoso LA, Merryman CF, Edelberg KE, Naids R, Kalsow C (1985) S-Antigen in the developing retina and pineal gland: a monoclonal antibody study. Invest Ophthalmol Vis Sci 26:561–567
Ermel AE, Brucher JM (1974) Arguments ultrastructureux en faveur de l'appartenance du medulloblastome a la lignee neuronale. Acta Neurol Belg 74:208–220
Frankfurter A, Binder LI, Rebhun LI (1986) Limited tissue distribution of a novel β-tubulin isoform (abstract). J Cell Biol 103:273a
Friede RL (1989) Gross and microscopic development of the central nervous system. In: Friede RL (ed) Developmental neuropathology, 2nd edn. Springer-Verlag, Berlin Heidelberg New York Tokyo, pp 2–20
Goldberg-Stern H, Gadoth N, Stern S, Cohen IJ, Zaizov R, Sandbank U (1991) The prognostic significance of glial fibrillary acidic protein staining in medulloblastoma. Cancer 68:568–573
Gould VE, Lee I, Wiedenmann B, Moll R, Chejfec G, Franke WW (1986) Synaptophysin: a novel marker for neurons, certain neuroendocrine cells, and their neoplasms. Hum Pathol 17:979–983
Gozes I (1982) Tubulin of the nervous system. Neurochem Int 4:101–120
Gray EG, Westrum LE, Burgoyne RD, Barron J (1982) Synaptic organisation and neuron microtubule distribution. Cell Tissue Res 226:579–588
Herman MM, Rubinstein LJ (1984) Divergen glial and neuronal differentiation in a cerebellar medulloblastoma in an organ culture system: in vitro occurrence of synaptic ribbons. Acta Neuropathol (Berl) 65:10–24
Herpers MJHM, Budka H (1985) Primitive neuroectodermal tumors including the medulloblastoma: glial differentiation signaled by immunoreactivity for GFAP is restricted to the pure desmoplastic medulloblastoma (“arachnoidal sarcoma of the cerebellum”). Clin Neuropathol 4:12–18
Katsetos CD, Liu HM, Zacks SI (1988) Immunohistochemical and ultrastructural observations on Homer Wright (neuroblastic) rosettes and the “pale islands” of human cerebellar medulloblastomas. Hum Pathol 19:1219–1227
Katsetos CD, Herman MM, Frankfurter A, Gass P, Collins P, Walker CC, Rosemberg S, Barnard RO, Rubinstein LJ (1989) Cerebellar desmoplastic medulloblastomas. A further immunohistochemical characterization of the reticulin-free pale islands. Arch Pathol Lab Med 113:1019–1029
Katsetos CD, Frankfurter A, Christakos S, Tsokos M, Valsamis MP Maker HS, Wolfe D, Di Rocco A, Bergland RM, Urich H (1991) Differential expression of neuronal class III β-tubulin isotype and calbidin D28K in the developing human cerebellar cortex and cerebellar neuroblastomas (“medulloblastomas”). J Neuropathol Exp Neurol 50:293
Kleihues P, Shibata T, Wiestler OD, Aguzzi A (1990) Morphological and immunohistochemical analysis of 330 medulloblastomas. Biwako Symposium on Brain Tumor Pathology. Biwako Hotel, Shiga, Japan, Sept 9, 1990, Japan Society of Brain Tumor Pathology, Abstr S-I-1, p 25
Korf HW, Czerwionka M, Reiner J, Schachenmayr W, Schalken JJ, De Grip W, Gery I (1987) Immunocytochemical evidence of molecular photoreceptor markers in cerebellar medulloblastomas. Cancer 60:1763–1766
Kumanishi T, Washiyama K, Watabe K, Sekiguchi K (1985) Glial fibrillary acidic protein in medulloblastomas. Acta Neuropathol (Berl) 67:1–5
Mannoji H, Takeshita I, Fukui M, Ohta M, Kitamura K (1981) Glial fibrillary acidic protein in medulloblastoma. Acta Neuropathol (Berl) 55:63–69
Packer RJ, Sutton LN, Rorke LB, Littman PA, Sposto R, Rosenstock JG, Bruce DA, Schut L (1984) Prognostic importance of cellular differentiation in medulloblastoma of childhood. J Neurosurg 61:296–301
Packer RJ, Tremblay JF, Rorke LB, Trojanowski JQ, Sutton LN, Bruce DB, Schut L (1985) Prognostic importance of expression of cytoskeletal proteins in childhood primitive neuroctodermal tumors (medulloblastoma). Ann Neurol 18:140
Pannese E (1974) The histogenesis of the spinal ganglia. Adv Anat Embryol Cell Biol 47:7–16
Perentes E (1991) Retinal S-antigen immunoreactivity in retinoblastomas, pineal parenchymal tumors, and medulloblastomas: an update. Brain Tumor Pathol (Jpn) 8:107–110
Perentes E, Rubinstein LJ, Herman MM, Donoso LA (1986) S-antigen immunoreactivity in human pineal glands and pineal parenchymal tumors. A monoclonal antibody study. Acta Neuropathol (Berl) 71:224–227
Perentes E, Maraziotis T, Qureshi SR (1991) Granular cell brain tumors of the laboratory rat: an immunohistochemical approach. Acta Neuropathol 82:112–117
Qureshi SR, Perentes E, Ettilin RA, Kolopp M, Prentice DE, Frankfurter A (1991) Morphologic and immunohistochemical characterization of Leydig cell tumor variants in Wistar rats. Toxicol Pathol 19:280–286
Rakic P (1971) Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electron microscopic study in Macacus rhesus. J Comp Neurol 141:283–312
Rakic P (1971) Guidance of neurons migrating to the fetal monkey neocortex. Brain Res 33:471–476
Rubinstein LJ (1985) Embryonal central neuroepithelial tumors and their differentiating potential. A cytogenetic view of a complex neuro-oncological problem. J Neurosurg 62:795–805
Schwechheimer K, Wiedenmann B, Franke WW (1987) Synaptophysin: a reliable marker for medulloblastomas. Virchows Arch [A] 411:53–59
Sternberger LA, Hardy PH Jr, Cuculis JJ, Meyer HG (1970) The unlabelled antibody enzyme method of immunohistochemistry. Preparation and properties of soluble antigen-antibody complex (horseradish-antihorseradish peroxidase) and its use in identification of spirochestes. J Histochem Cytochem 18:315–333
Tennyson V (1970) The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J Cell Biol 44:62–79
Wacker WB, Donoso LA, Kalsow CM, Yankeelov JA, Organisciak DT (1977) Experimental allergic uveitis. Isolation, characterization and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol 119:1949–1958
Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE (1986) Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci USA 83:3500–3504
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maraziotis, T., Perentes, E., Karamitopoulou, E. et al. Neuron-associated class III β-tubulin isotype, retinal S-antigen, synaptophysin, and glial fibrillary acidic protein in human medulloblastomas: a clinicopathological analysis of 36 cases. Acta Neuropathol 84, 355–363 (1992). https://doi.org/10.1007/BF00227661
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227661