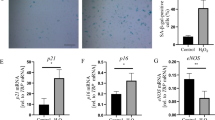
Summary
To study the effect of haemodynamic stress on the morphological differentiation of pseudointima, the ultrastructure of the cells lining normally shaped and aneurysmal polyurethane vascular prostheses implanted into the abdominal aorta of rats was examined. In the normally shaped vascular prostheses the pseudointima was composed of several layers of smooth muscle cells, which varied in differentiation from normal smooth muscle cells to myofibroblasts, and which were lined by a continuous sheet of endothelial cells. In the aneurysmal vascular prostheses, a pseudointima, composed of only layers of smooth muscle cells had developed. Those smooth muscle cells which lined the lumen had a typical morphology: they were polygonal, flat cells of unequal size, with a distinct organelle-free zone, containing myofilaments, at the luminal peripheral cytoplasmic side. The other smooth muscle cells varied in differentiation from normal smooth muscle cells to myofibroblasts. Under severe haemodynamic stresses, such as occur in the aneurysmal vascular prostheses, the regeneration of endothelial cells is impaired and smooth muscle cells undergo morphological changes to form a pseudoendothelial lining.
Similar content being viewed by others
References
Abbott WM, Cambria RP (1982) Control of physical characteristics (elasticity and compliance) of vascular grafts. In: Stanley JC, Burkel WE, Lindenauer SM, Bartlett RH, Turcotte JG (eds) Biologic and synthetic vascular prostheses. Grune and Stratton, New York, pp 131–152
Bell FP, Day AJ, Gent M, Schwartz SM (1975) Differing patterns of cholesterol accumulation and 3-H-cholesterol influx in areas of the cholesterol-fed pig aorta identified by evans blue dye. Exp Mol Pathol 22:366–375
Berger K, Sauvage LR, Rao AM, Wood SJ (1972) Healing of arterial prostheses in man: Its incompleteness. Ann Surg 175:118–127
Bjorkerud S, Bondjers G (1971) Arterial repair and atherosclerosis after mechanical injury. Part 2. Tissue response after induction of a total local necrosis (deep long injury). Atherosclerosis 14:259–276
Bjorkerud S, Bondjers G (1973) Arterial repair and atherosclerosis after mechanical injury. Part 5. Tissue response after induction of a large superficial transverse injury. Atherosclerosis 18:235–255
Clagett GP, Robinowitz M, Maddox Y, Langloss JM, Ramwell PW (1982) The antithrombogenic nature of vascular prosthetic pseudointima. Surgery 91:87–94
Clowes AW, Gown AM, Hanson SR, Reidy MA (1985) Mechanism of arterial graft failure: I. Role of cellular proliferation in early healing of PTFE prostheses. Am J Pathol 118:43–54
Clowes AW, Kirkman TR, Reidy MA (1986) Mechanism of arterial graft failure: III. Rapid transmural capillary ingrowth provides a source of intimal endothelium and smooth muscle in porous PTFE prostheses. Am J Pathol 123:220–230
Dewey CF (1984) Effects of fluid flow on living vascular cells. J Biochem Eng 106:31–35
Fishman JA, Ryan GB, Karnovsky MJ (1974) Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest 32:339–351
Fry DL (1968) Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res 22:165–197
Fry DL (1976) Hemodynamic Forces in atherogenesis. In: Steinberg P (ed) Cerebrovascular diseases. Raven Press, New York, pp 77–95
Gerrity RG, Cliff WJ (1975) The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Lab Invest 32:585–600
Gospodarowicz D, Vlodavsky I, Fielding P, Birdwell CR (1978) The effects of the epidermal and fibroblast growth factors upon cell proliferation using vascular and corneal endothelial cells as a model. In: Littlefield JW, De Grouchy J (eds) Birth Defects. Exerpta Medica, Amsterdam, pp 233–271
Greenhill NS, Stehbens WE (1982) Scanning electron microscopic study of the inner surface of experimental aneurysms in rabbits. Atherosclerosis 45:319–330
Hinek A, Thyberg J (1977) Electron microscopic observations on the formation of elastic fibers in primary cultures of aortic smooth muscle cells. J Ultrastruct Res 60:395–401
Hulstaert CE, Kalicharan D, Hardonk MJ (1983) Cytochemical demonstration of phosphatases in the rat liver by a ceriumbased method in combination with osmium tetroxide and potassium ferrocyanide postfixation. Histochemistry 78:71–79
Imperato AM, Bracco A, Kim GE, Zeff R (1972) Intimal and neointimal fibrous proliferation causing failure of arterial reconstructions. Surgery 72:1007–1017
Jackman RW (1982) Persistence of axial orientation cues in regenerating intima of cultered aortic explants. Nature 296:80–83
Joris I, Zand T, Majno G (1981) Hydrodynamic injury of the endothelium in acute aortic stenosis. Am J Pathol 39:367–381
Kadish JL, Butterfield CE, Folkman J (1979) The effect of fibrin on cultured vascular endothelial cells. Tissue Cell 11:99–108
Langille BL, Reidy MA, Kline RL (1986) Injury and repair of endothelium at sites of flow disturbances near aortic coarctations in rabbits. Arteriosclerosis 6:146–154
Van der Lei B, Darius H, Schror K, Molenaar I, Nieuwenhuis P, Wildevuur ChRH (1984) Improved neoendothelialization of small caliber vascular grafts. Life Support Syst 2 [Suppl] 1:332–334
Van der Lei B, Darius H, Schror K, Nieuwenhuis P, Molenaar I, Wildevuur ChRH (1985a) Arterial wall regeneration in small caliber vascular grafts in rats. Neoendothelial healing and prostacyclin production. J Thorac Cardiovasc Surg 90:378–386
Van der Lei B, Bartels HL, Nieuwenhuis P, Wildevuur ChRH (1985b) Microporous, compliant, biodegradable vascular grafts for the regeneration of the arterial wall in rat abdominal aorta. Surgery 98:955–963
Van der Lei B, Wildevuur ChRH, Nieuwenhuis P, Blaauw EH, Dijk F, Hulstaert CE, Molenaar I (1985c) Regeneration of the arterial wall in microporous, compliant, biodegradable, vascular grafts after implantation into the rat abdominal aorta: Ultrastructural observations. Cell Tissue Res 242:569–578
Van der Lei B, Wildevuur ChRH, Nieuwenhuis P (1985d) Mechanical stimulation of smooth muscle cells by arterial pulsations. An important stimulus for the formation of elastic laminae in arterial tissue. Cell Biol Int Rep 9:2
Van der Lei B (1986a) From synthetic vascular graft to new artery. Thesis, University of Groningen, The Netherlands
Van der Lei B, Wildevuur ChRH, Nieuwenhuis P (1986b) Compliance and biodegradation of vascular grafts stimulate the regeneration of elastic laminae in neoarterial tissue: An experimental study in rats. Surgery 99:45–52
Van der Lei B, Wildevuur ChRH, Dijk F, Blaauw EH, Molenaar I, Nieuwenhuis P (1987) Sequential studies of arterial wall regeneration in microporous, compliant, biodegradable small-caliber vascular grafts in rats. J Thorac Cardiovasc Surg 93:695–707
Levesque MJ, Liepsch D, Moravec S, Nerem RM (1986) Correlation of endothelial cell shape and wall shear stress in a stenoses dog aorta. Arteriosclerosis 6:220–229
Madri JA, Stenn KS (1982) Aortic endothelial cell migration. I. Matrix requirements and composition. Am J Pathol 106:180–186
Malckzak HT, Buck RC (1977) Regeneration of endothelium in rat aorta after local freezing. A scanning electron microscopic study. Am J Pathol 86:133–141
Minick CR, Stemerman MB, Insull W Jr (1979) Role of endothelium and hypercholesterolemia in intimal thickening and lipid accumulation. Am J Pathol 95:131–158
Porter JM (1986) Arterial substitutes. In: Sabiston DC (ed) Textbook of Surgery: The biological basis of modern surgical practice. WB Saunders Company, Philadelphia, pp 1778–1793
Reidy MA, Bowyer DE (1977) Scanning electron microscopy of arteries. The morphology of aortic endothelium in haemodynamically stressed areas associated with branches. Atherosclerosis 26:181–194
Reidy MA, Langille BL (1980) The effect of local blood flow patterns on endothelial cell morphology. Exp Mol Pathol 32:276–289
Reidy MA, Schwartz SM (1981) Endothelial regeneration. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest 44:301–308
Rogers KM, Merrilees MJ, Stehbens WE (1985) The effect of haemodynamic stress on the glycosaminoglycan content of blood vessel walls of experimental aneurysms and arteriovenous fistulae. Atherosclerosis 58:139–148
Ross R (1983) Atherosclerosis: A question of endothelial integrity and growth control of smooth muscle, The Harvey Lectures, Series 77, 1981–1982. Academic Press, New York, pp 161–182
Saba HI, Hartman RC, Saba SR (1978) Effects of polymorphonuclear leukocytes on endothelial cell growth. Thromb Res 12:397–407
Sauvage LR, Berger KE, Wood SJ, Yates SG, Smith JC, Mansfield PB (1974) Interspecies healing of porous arterial prostheses: Observations, 1960 to 1974. Arch Surg 109:698–705
Schwartz SM, Haudenschild CC, Eddy EM (1978) Endothelial regeneration. I. Quantitative analysis of initial stages of endothelial regeneration in rat aortic intima. Lab Invest 38:568–580
Sean Moore MB, Belbeck LW, Richardson M, Taylor W (1982) Lipid accumulation in the neointima formed in normally fed rabbits in response to one or six removals of the aortic endothelium. Lab Invest 47:37–42
Stanley JC, Burkel WE, Lindenauer SM, Bartlett RH, Turcotte JG (1982) Biologic and synthetic vascular prostheses. Grune and Stratton, New York
Stemerman MB, Spaet TH, Pitlick F, Cintron J, Lejnieks I, Tiell ML (1977) Intimal healing. The pattern of reendothelialization and intimal thickening. Am J Pathol 87:125–142
Wall RT, Harker LA, Quadracci LJ (1978) Factors influencing endothelial cell proliferation in vitro. J Cell Physiol 96:203–214
White CE, Fujiwara K, Shefton EJ, Dewey CF, Gimbrone MA (1982) Fluid shear stress influences cell shape and cytoskeletal organization in cultured vascular endothelium. Fed Proc 41:321
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Lei, B., Schakenraad, J.M. Differentiation of vascular pseudointima under normal and disturbed blood flow conditions: Ultrastructural observations in the rat. Cell Tissue Res. 254, 647–654 (1988). https://doi.org/10.1007/BF00226515
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00226515