Summary
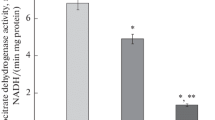
The influence of starvation on renal carbohydrate metabolism was studied in the proximal and distal fragments of the nephron. Starvation induced a double and opposite adaptation mechanism in both fractions of the renal tubule. In renal proximal tubules, the gluconeogenic flux was stimulated progressively during a period of 48 hours of starvation (2.15 fold), due, in part, to a significant increase in the fructose 1,6-bisphosphatase and phosphoenolpyruvate carboxykinase activities although with different characteristics. Fructose 1,6-bisphosphatase activity from this tubular fragment increased only at subsaturating subtrate concentration (68%) which involved a significant decrease in the Km (35%) for fructose 1,6-bisphosphate while there was no change in Vmax. This behaviour clearly indicates that it is related to modifications in the activity of the preexistent enzyme in the cell. Proximal phosphoenolpyruvate carboxykinase activity increased proportionally at both substrate concentrations (86 and 89% respectively) which brought about changes in Vmax without changes in Kin, all of which are in accordance with variations in the cellular levels of the enzyme. In the renal distal tubules, the glycolytic capacity drastically decreased throughout the starvation time. At 48 hours 65% of inhibition was shown. We have found a short term regulation of phosphofructokinase activity by starvation which involves an increase in Km (2.2 fold) without changes in Vmax, as a result of these kinetic changes, an inactivation of phosphofructokinase was detected at subsaturating concentration of fructose 6-phosphate. On the contrary, this nutritional state did not modify the kinetic behaviour of renal pyruvate kinase. Finally, neither proximal glycolytic nor distal gluconeogenic capacities and related enzymes activities were changed during starvation.
Similar content being viewed by others
References
Krebs HA, Bennett DAH, De Gasquet P, Gascoyne T, Yoshida T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat kidney cortex slices. Biochem J 86:22–27, 1963
Churchill PC, Belloni FL, Churchill MC: Net renal glucose release in rat. Am J Physiol 225:528–531, 1973
Kida K, Nakajo S, Kamiya F, Toyama Y, Nishio T, Nakagawa H: Renal net glucose release in vivo and its contribution to blood glucose in rats. J Clin Invest 62:721–726, 1978
Owen OE, Felig P, Morgan AP, Wahren J, Cahill CF Jr: Liver and kidney metabolism during prolonged starvation. J Clin Invest 48:574–583, 1969
Lupiáñez JA, Faus MJ, Muñoz-Clares R, Sánchez-Medina F: Stimulation of rat kidney gluconeogenesis ability by inhibition of liver gluconeogenesis. FEBS Lett. 61:277–281, 1976
Faus MJ, Lupiañez JA, Vargas A, Sánchez-Medina F: Induction of rat kidney gluconeogenesis during acute liver intoxication by carbon tetrachloride. Biochem J 174:461–468, 1978
Brinkman A, Katz N, Sasse D, Jungermann K: Increase of the gluconeogenic and decrease of the glycolytic capacity of rat liver with a change of the metabolic zonation after partial hepatectomy. Hoppe-Seyler's Z Physiol Chem 359:1561–1571, 1978
Sanchez-Pozo A, Lupiáñez JA, Corno A, Pita ML, Sánchez-Medina F: Differential effects of L-tryptophan administration on hepatic and renal gluconeogenesis in rats. Molecular Physiology 3:143–150, 1983
Iynedjian PB, Ballard FJ, Hanson RW: The regulation of phosphoenolpyruvate carboxykinase (GTP) synthesis in rat kidney cortex. The role of acid base balance and glucocorticoids. J Biol Chem 250:5596–5603, 1975
Pogson CI, Longshaw ID, Roobol A, Smith SH, Alleyne GAO: Phosphoenolpyruvate carboxykinase and renal gluconeogenesis. In: RW Hanson and MA Mehlman (eds) Gluconeogenesis. Its regulation in mammalian species. John Wiley and Sons, New York, pp 335–368, 1976
Cimbala MA, Lamers WH, Nelson K, Monahan JE, Yoo-Warren H, Hanson RW: Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney effects of insulin and cyclic AMP. J Biol Chem 257:7629–7636, 1982
Guder WG, Ross BD: Enzyme distribution along the nephron. Kidney Int 26:101–111, 1984
Vinay P, Gougoux A, Lemieux G: Isolation of a pure suspension of rat proximal tubules. Am J Physiol 241:17403–17411, 1981
Mendicino J, Vasarhely F: Renal D-fructose 1,6-diphosphatase. J Biol Chem 238:3528–3534, 1963
Ballard FJ, Hansen RW: Phosphoenolpyruvate carboxykinase and pyruvate carboxylase in developing rat liver. Biochem J 104:866–871, 1967
Castaño TG, Nieto A, Feliu JE: Inactivation of phosphofructokinase by glucagon in rat hepatocytes. J Biol Chem 254:5576–5579, 1979
Carbonell J, Feliu JE, Marco R, Sols A: Pyruvate kinase classes of regulatory isoenzymes in mammalian tissues. Eur J Biochem 37:148–150, 1973
Lowry OH, Rosebrough NJ, Farr JL, Randall RJ: Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275, 1951
Bradford M: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254, 1976
Bergmeyer HU, Bernt E: Determination with glucose oxidase and peroxidase. In: HU Bergmeyer (ed) Methods of Enzymatic Analysis. Verlag Chemie Academic Press Inc, London and New York, pp 1205–1212, 1974
Gawehn K, Bergmeyer HU: L-(+)-lactate. Determination with lactate dehydrogenase and NAD. In: HU Bergmeyer (ed) Methods of Enzymatic Analysis. Verlag Chemie Academic Press Inc, London and New York, pp 1492–1495, 1974
Czok R, Lamprecht W. Pyruvate, phosphoenolpyruvate and D-glycerate-2-phosphate. In: HU Bergmeyer (ed) Methods of Enzymatic Analysis. Verlag Chemie Academic Press Inc, London and New York, pp 1446–1451, 1974
Azzout B, Peret J: Development of gluconeogenesis from dihydroxyacetone in rat hepatocytes during a feeding cycle and starvation. Biochem J 218:975–981, 1984
Atkins GL, Nimmo LA: A comparison of seven methods for fitting the Michaelis-Menten equation. Biochem J 149:775–777, 1975
Ross BD, Espinal J, Silva P: Glucose metabolism in renal tubular function. Kidney Int 29:54–67, 1986
Klein KL, Wang MS, Torikai S, Davidson WD, Kurokawa K: Substrate oxidation by isolated single nephron segments of the rat. Kidney Int 20:29–35, 1981
Heitmann RN, Bergman EN: Glutamate interconversions and glucogenicity in the sheep. Am J Physiol 241:E465-E472, 1981
Moore RE, Hansen JB, Lardy HA, Veneziale CM: The development and application of a radioimmunoassay for rat phosphoenolpyruvate carboxykinase. J Biol Chem 257:12546–12552, 1982
Dunaway GA: A review of animal phosphofructokinase isozymes with an emphasis on their physiological role. Mol Cell Biochem 52:75–91, 1983
Schering B, Reinacher M, Schoner W: Localization and role of pyruvate kinase isoenzymes in the regulation of carbohydrate metabolism and pyruvate recycling in rat kidney cortex. Biochim Biophys Acta 881:62–71, 1986
Krebs HA, Eggleston LV: The role of pyruvate kinase in the regulation of gluconeogenesis. Biochem J 94:3C-4C, 1965
Poole GP, Bloxham DP: The turnover of L-type pyruvate kinase in cultured rat hepatocytes. Biochem J 204:89–95, 1982
Van Berkel TIC, Kruijt JK, Van den Berg GB, Koster JF: Difference in the effect of glucagon and starvation upon L type pyruvate kinase from rat liver. Eur J Biochem 92:553–561, 1978
Kohl EA, Cottam GL: Alteration in liver pyruvate kinase protein and catalytic activity upon starvation and refeeding. Arch Biochem Biophys 176:671–682, 1976
Cimbala MA, Lau D, Daigneault JF: Starvation and feeding with a high-carbohydrate diet induce changes in the specific activity of rat hepatic pyruvate kinase. Biochem J 226:299–303, 1985
Ferré P, Pégorier JP, Williamson DH, Girard J: Interactions in vivo between oxidation of non-esterified fatty acids and gluconeogenesis in the newborn rat. Biochem J 182:593–598, 1979
Denton RM, Halestrap AP: Regulation of pyruvate metabolism in mammalian tissues. Essays Biochem 15:37–77, 1979
Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF Jr: Glucagon levels and metabolic effects in fasting man. J Clin Invest 49:2256–2270, 1970
Silva P, Ross B, Spokes K: Competition between sodium reabsorption and gluconeogenesis in kidneys of steroid-treated rats. Am J Physiol 238:17290–17295, 1980
Hue L, Blackmore P, Exton JH: Fructose 2,6-bisphosphate. Hormonal regulation and mechanism of its formation in liver J Biol Chem 256:8900–8903, 1981
Pilkis SJ, Chrisman TD, Burgress B, McGrane M, Colosia A, Pilkis J, Claus TH, El-Maghrabi MR: Rat hepatic 6phosphofructo-2-kinase/fructose 2,6-bisphosphatase: a unique bifunctional enzyme. Adv Enzyme Regul 21:147–173, 1983
Van-Schaftingen E, Hers HG: Inhibition of fructose 1,6-bisphosphatase by fructose 2,6-bisphosphate. Proc Natl Acad Sci USA 78:2861–2863, 1981
Uyeda K, Furuya E, Sherry AD: The structure of ‘activation factor’ for phosphofructokinase. J Biol Chem 256:8679–8684, 1981
Brosnan JT, McPhee P, Hall B, Parry DM: Renal glutamine metabolism in rats fed high-protein diets. Am J Physiol 235:E261-E265, 1978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
García-Salguero, L., Lupiáñez, J.A. Metabolic adaptation of the renal carbohydrate metabolism. I. Effects of starvation on the gluconeogenic and glycolytic fluxes in the proximal and distal renal tubules. Mol Cell Biochem 83, 167–178 (1988). https://doi.org/10.1007/BF00226144
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00226144