Summary
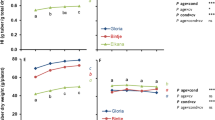
The objective of this study was to separate and determine effects on the field performance of transgenic potatoes that originate from the tissue culture process of transformation and from the genes inserted. The constructs introduced contained the reporter gene for betaglucuronidase (GUS) under the control of the patatin promoter (four different constructs) and the neomycin phosphotransferase gene under the control of the nopaline synthase promoter. Both genes might be expected to have a neutral effect on plant phenotype. The field performance of transgenic plants (70 independent transformants) was compared with non-transgenic plants regenerated from tuber discs by adventitious shoot formation and from shoot cultures established from tuber nodal cuttings. Plants from all three treatments were grown in a field trial from previously field-grown tubers, and plant performance was measured in terms of plant height at flowering, weight of tubers, number of tubers, weight of large tubers and number of large tubers. There was evidence of somaclonal variation among the transgenic plants; mean values for all characters were significantly lower and variances generally higher than from plants derived from nodal shoot cultures. A similar change in means and variances was observed for the non-transgenic tuber-disc regenerants when compared with shoot culture plants. Plant height, tuber weight and tuber number were, however, significantly lower in transgenic plants than in tuber-disc regenerants, suggesting an effect on plant performance either of the tissue culture process used for transformation or of the genes inserted. There were significant differences between constructs for all five plant characters. The construct with the smallest segment of patatin promoter and the lowest level of tuber specificity for GUS expression had the lowest values for all five characters. It is proposed that the nature of GUS expression is influencing plant performance. There was no indication that the NPTII gene, used widely in plant transformation, has any substantial effect on plant performance in the field.
Similar content being viewed by others
References
Allard RW (1960) Principles of plant breeding. John Wiley and Sons, New York.
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721.
Blundy KS, Blundy MAC, Carter D, Wilson F, Park WD, Burrell MM (1991) The expression of class I patatin gene fusions in transgenic potato varies with both gene and cultivar. Plant Mol Biol 16:153–160.
Clark MF, Adams AN (1977) Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses J Gen Virol 34:475–483.
D'Halluin K, Botterman J, De Greef W (1990) Engineering of herbicide resistant alfalfa and evaluation under field conditions. Crop Sci 30:866–871.
Dale PJ, McQueen AP, Sweatman SM, Wray RW (1986) Annual Report. Plant Breeding Institute, Cambridge, UK, pp 51–52.
De Greef W, Delon R, De Block M, Leemans J, Botterman J (1989) Evaluation of herbicide resistance in transgenic crops under field conditions. Biotechnology 7:61–64.
Delannay X, LaVallee BJ, Proksch RK, Fuchs RL, Sims SR, Greenplate JT, Marrone PG, Dodson RB, Augustine JJ, Layton JG, Fischhof DA (1989) Field performance of transgenic tomato plants expressing the Bacillus thuringiensis var. Kurstaki insect control protein. Biotechnology 7:1265–1269.
Errampalli D, Patton D, Castle L, Mickelson L, Hansen K, Schnall J, Feldman K, Meinke D (1991) Embryonic lethals and T-DNA insertional mutagenesis in Arabidopsis. The Plant Cell 3:149–157.
Feldmann KA, Marks MD (1987) Agrobacterium-mediated transformation of germinating seeds of Arabidopsis thaliana: a non-tissue culture approach. Mol Gen Genet 208:1–9.
Feldmann KA, Marks MD, Christianson ML, Quatrano RS (1989) A dwarf mutant of Arabidopsis generated by T-DNA insertion mutagenesis. Science 243:1351–1354.
Fincham JRS (1983) Genetics. John Wiley and Sons, Bristol.
Hobbs SLA, Kpodar P, DeLong CMO (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15:851–864.
Jefferson RA (1989) The GUS reporter gene system. Nature 342:837–838.
Jefferson RA (1990) New approaches for agricultural molecular biology: from single cells to field analysis. In: Gustafson JP (ed) Gene manipulation in plant improvement II. Plenum Press, New York, pp 365–400.
Jefferson R, Goldsbrough A, Bevan M (1990) Transcriptional regulation of a patatin-1 gene in potato. Plant Mol Biol 14:995–1006.
Karp A (1991) On the current understanding of somaclonal variation. Oxford Surveys Plant Mol Cell Biol 7:1–58.
Karp A, Bright SWJ (1985) On the causes and origins of somaclonal variation. Oxford Surveys Plant Mol Cell Biol 2:199–234.
Larkin PJ, Scowcroft WR (1981) Somaclonal variation — a novel source of variation from cell cultures for plant improvement. Theor Appl Genet 60:197–214.
Lycett GW, Grierson D (1990) (eds) Genetic engineering of crop plants. Butterworth, London.
McHughen A, Holm F (1991) Herbicide resistant transgenic flax field test: agronomic performance in normal and sulfonyl-urea-containing soils. Euphytica 55:49–56.
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant 15:473–497.
Nelson RS, McCormick SM, Delannay X, Dube P, Layton A, Anderson EJ, Kaniewska M, Proksch RK, Horsch R, Rogers SG, Fraley RT, Beachy RN (1988) Virus tolerance, plant growth, and field performance of transgenic tomato plants expressing coat protein from tobacco mosaic virus. Biotechnology 6:403–409.
Rietveld RC, Hasegawa PK, Bressan RA (1991) Somaclonal variation in tuber disc-derived populations of potato I. Evidence of genetic stability across tuber generations and diverse locations. Theor Appl Genet 82:430–440.
Sheerman S, Bevan MW (1988) A rapid transformation method for Solanum tuberosum using binary Agrobacterium tumefaciens vectors. Plant Cell Rep 7:13–16.
Snedecor GW, Cochran WG (1990) Statistical methods. Iowa State University Press, Iowa, USA.
Westcott RJ, Henshaw GG, Grout BWW (1977) Tissue culture methods and germplasm storage in potato. ACTA Horticul 78:45–49.
Wheeler VA, Evans NE, Foulger D, Webb KJ, Karp A, Franklin J, Bright SWJ (1985) Shoot formation from explant cultures of fourteen potato cultivars and studies of the cytology and morphology of regenerated plants. Ann Bot 55:309–320.
Author information
Authors and Affiliations
Additional information
Communicated by J. W. Snape
Rights and permissions
About this article
Cite this article
Dale, P.J., McPartlan, H.C. Field performance of transgenic potato plants compared with controls regenerated from tuber discs and shoot cuttings. Theoret. Appl. Genetics 84, 585–591 (1992). https://doi.org/10.1007/BF00224156
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00224156