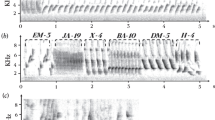
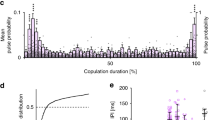
Summary
In Xenopus laevis, adult males but not females produce courtship songs comprised of rapid trills. Two experiments were conducted to determine whether male-typical singing could be induced in females. At 6 different juvenile stages, male and female frogs were gonadectomized and implanted with testes, grown to sexual maturity, and tested for vocal behavior. All frogs with functional testicular implants sang; females sang as much as males. The frequency spectra of the clicks within trills were fully masculinized in females implanted at PM0, PM1, and PM2. There were deficiencies in song quality in females implanted late in juvenile life. Females receiving testis implants at PM3, PM4, and PM5 did not produce clicks with masculine spectral qualities. In a concurrent experiment, adult males and females were gonadectomized and implanted with testes or silicone tubes containing testosterone proprionate. When tested for vocal behavior 10 to 15 months after implantation, 8/10 androgen-treated males, 3/12 androgen-treated females, 5/5 testes-implanted males, and 2/4 testes-implanted females sang. The females that did sing spent much less time singing than males. The click rates of females were uniformly slower than males and no female produced clicks with a masculine frequency spectrum. Thus, testicular secretions can induce male-typical singing in females until late in juvenile development. However, females exhibit a progressive decline in vocal potential with increasing age, culminating in an almost complete loss of singing ability by adulthood.
Similar content being viewed by others
Abbreviations
- FFT :
-
fast Fourier transform
- ICI :
-
inter-click interval
- PM :
-
post-metamorphic
- TP :
-
testosterone proprionate
References
Adkins-Regan E, Ascenzi M (1987) Social and sexual behavior of male and female zebra finches treated with estradiol during the nestling period. Anim Behav 35:1100–1112
Adkins-Regan E, Ascenzi M (1990) Sexual differentiation of behavior in the zebra finch: effect of early gonadectomy or androgen treatment. Horm Behav 24:114–127
Adkins-Regan E, Orgeur P, Signoret JP (1989) Sexual differentiation of reproductive behavior in pigs: Defeminizing effects of prepubertal estradiol. Horm Behav 23:290–303
Arnold AP, Breedlove SM (1985) Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Horm Behav 19:469–498
Arnold AP, Schlinger BA (1991) The importance of brain aromatization for sexual differentiation of zebra finch brain. Soc Neurosci Abstr 17:1410
Gurney ME (1981) Hormonal control of cell form and number in the zebra finch song system. J Neurosci 1:658–673
Gurney ME (1982) Behavioral correlates of sexual differentiation in the zebra finch song system. Brain Res 231:153–172
Gurney M, Konishi M (1980) Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science 208:1380–1383
Hannigan P, Kelley DB (1986) Androgen-induced alterations in vocalization of female Xenopus laevis: modifiability and constraints. J Comp Physiol A 158:517–527
Kelley DB (1988) Sexually dimorphic behavior. Annu Rev Neurosci 11:225–251
Kelley D, Morrell J, Pfaff D (1975) Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis I. Testosterone J Comp Neurol 164:63–78
Lambdin L, Kelley D (1986) Organization and activation of sexually dimorphic vocalizations: androgen levels in developing and adult Xenopus laevis. Soc Neurosci Abstr 12:1213
Marin M, Tobias M, Kelley D (1990) Hormone sensitive stages in the sexual differentiation of laryngeal muscle fiber number in Xenopus laevis. Development 110:703–712
Pfeiffer C (1936) Sexual differences in the hypophyses and their determination by the gonads. Am J Anat 58:195–225
Phoenix C, Goy R, Gerall A, Young W (1959) Organizing action of prenatally administered testosterone proprionate on the tissues mediated mating behavior in the female guinea pig. Endocrinology 65:369–382
Picker ND (1983) Hormonal induction of the aquatic phonotactic response of Xenopus. Behaviour 86:74–90
Ridewood, W (1898) On the structure and development of the hyobranchial skeleton and larynx in Xenopus and Pipa; with remarks on the affinities of the Aglossa. Linn Soc J Zool 26:53–128
Sassoon D, Kelley DB (1986) The sexually dimorphic larynx of Xenopus laevis: development and androgen regulation. Am J Anat 177:457–472
Sassoon D, Gray G, Kelley D (1987) Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J Neurosci 7:3198–3206
Schmidt RS (1976) Neural correlates of frog calling. Isolated brain-stem. J Comp Physiol 108:99–113
Siegel S (1956) Nonparametric statistics for the behavioral sciences. McGraw-Hill, New York
Tobias ML, Kelley DB (1987) Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J Neurosci 7:3191–3197
Tobias M, Marin M, Kelley D (1991a) Development of functional sex differences in the larynx of Xenopus laevis. Dev Biol 147:251–259
Tobias ML, Marin ML, Kelley DB (1991b) Temporal constraints on androgen directed laryngeal masculinization in Xenopus laevis. Dev Biol 147:260–270
Wetzel D, Kelley D (1983) Androgen and gonadotropin control of the mate calls of male South African clawed frogs, Xenopus laevis. Horm Behav 17:388–404
Wetzel D, Haerter U, Kelley D (1985) A proposed neural pathway for vocalization in South African clawed frogs, Xenopus laevis. J Comp Physiol A 157:749–761
Weintraub A, Kelley DB, Bockman R (1985) Prostaglandin E2 induces receptive behaviors in female Xenopus laevis. Horm Behav 19:386–399
Yager D (1992a) A unique sound production system in the pipid anuran Xenopus borealis. Zool J Linn Soc 104:351–375
Yager D (1992b) Underwater acoustic communication in Xenopus borealis. Bioacoustics 4:1–24
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watson, J.T., Kelley, D.B. Testicular masculinization of vocal behavior in juvenile female Xenopus laevis reveals sensitive periods for song duration, rate, and frequency spectra. J Comp Physiol A 171, 343–350 (1992). https://doi.org/10.1007/BF00223964
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00223964