Abstract
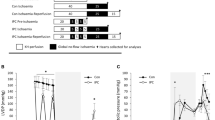
In this study we prepared sarcolemmal fractions from bovine and rat hearts; their Na+K+ ATPase activities, measured in the presence of saponin to unmask latent Na+K+ ATPase, were 59.4 and 48.8 µ mol Pi/mg protein · h, respectively. The rate of Na+dependent Ca2+ uptake was linear for the first 10 s and a plateau was reached in 3 min. Oxidation by free radical generation either with H2O2, FeSO4 plus DTT or xanthine oxidase plus hypoxanthine stimulated Na+/Ca2+ exchange in a time-dependent manner. The stimulation was abolished by deferoxamine or o-phenanthroline. By contrast, oxidation by HOCI inhibited Na+/Ca2+ exchange in proportion to its concentration, and this inhibition was antagonized by DTT. DTT alone had no effect on the exchange. Insulin stimulated Na+/Ca2+ exchange, its maximal effect was attained after 30min incubation with 100 µ units/ml. N-ethylmaleimide inhibited the exchange both in the presence and in the absence of insulin. Sarcolemmal fractions prepared from hearts of alloxan-treated, acutely diabetic rats showed a significant decrease in Na+/Ca2+ exchange. Addition of insulin in vitro significantly stimulated Na+/Ca2+ exchange of both diabetic and control groups. The results indicate that sarcolemmal Na+/Ca2+ exchange function is modulated by oxidation-reduction states and by the presence of insulin.
Similar content being viewed by others
References
Takahashi K, Kako KJ: Ischemia-induced changes in sarcolemmal Na+K+ ATPase, K+ pNPPase, sialic acid and phospholipid in the dog and effects of the Nisoldipine and chlorpromazine treatment. Biochem Med 31:271–286, 1984
Kako KJ, Kato M, Matsuoka T, Mustapha A: The depression of membrane-bound Na+K+ ATPase activity induced by free radicals and by ischemia of the kidney. Am J Physiol 254:C330-C337 1988
Kato M, Kako KJ: Effect of N-(2-mercaptopropionyl)glycine on ischemic-reperfused dog kidney in vivo and membrane preparation in vitro. Mol Cell Biochem 78:151–159 (1987)
Dhalla NS, Pierce GN, Panagia V, Signal PK, Beamish RE: Calcium movements in relation to heart function. Basic Res Cardiol 77:117–139, 1982
Dhalla NS, Pierce GN, Innes IR, Beamish RE: Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol 1:263–281, 1985
Ganguly PK, Pierce GN, Dhalla KS, Dballa NS: Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244:E528–535, 1983
Pierce GN, Kutryk MJB, Dhalla NS: Alterations in Ca binding by and composition of the cardiac sarcolemmal membrane in chronic diabetes. Proc Nat Acad Sci US 80:5412–5516, 1983
Makino N, Dhalla KS, Elimban V, Dhalla NS: Sarcolemmal Ca transport in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol 253:E202–207, 1987
Heyliger CE, Prakash A, McNeil JH: Alterations in cardiac sarcolemmal Ca pump activity during diabetes mellitus. Am J Physiol 252:H540–544, 1987
Pierce GN, Dhalla NS: Cardiac myofibrillar ATPase activity in diabetic rats. J Mol Cell Cardiol 13:1063–1069, 1981
Ganguly PK, Rice KM, Panagia V, Dhalla NS: Sarcolemmal phosphatidylethanolamine N-methylation in diabetic cardiomyopathy. Circ Res 55:504–512, 1984
Czech MP, Lawrence Jr JC, Lynn WS: Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell bexose transport by insulin. Proc Nat Acad Sci USA 71:4173–4177, 1974
Ramasarma T: Generation of H2O2 in biomembranes. Biochim Biophys Acta 694:69–93, 1982
Hayes GR, Lockwood DH: Role of insulin receptor phosphorylation in the the insulinomimetic effects of hydrogen peroxide. Proc Nat Acad Sic USA 84:8115–8119, 1987
Kozka IJ, Gould MK: Inhibitory effects of Nethylmaleimide on insulin- and oxidant-stimulated sugar transport and on 125I-labelled insulin binding by rat soleus muscle. Biochim Biophys Acta 797:212–220, 1984
Pershadsingh HA, Shade DL, Delfert DM, McDonald JM: Chelation of intracellular calcium blocks insulin arction in the adipocyte. Proc Nat Acad Sci USA 84:1025–1029, 1987
Takasu N, Yamada T, Shimizu Y: Generation of H202 is regulated by cytoplasmic free calcium in cultured porcine thyroid cells. Biochem Biohphys Res Comm 148:1527–1532, 1987
Orrenius S and Bellomo G: Toxicological implications of perturbation of Ca2+ homeostasis in hepatocytes. Calcium Cell Funct. 4:185–208, 1986
Carafoli E, Longoni S: The plasma membrane in the control of the signaling function of calcium. In IJ Mandel and DC Eaton (eds). Cell Calcium and the Control of Membrane Transport, Rockefeller Univ. Press, New York, 21–30, 1986
Parker JC: Diamide stimulates calcium-sodium exchange in dog red blood cells. Am J Physiol 253:C580–587, 1987
Carpentier JL, Gordon P, Robert A, Orci L: Internalization of polypeptide hormones and receptor recycling. Experientia 42:734–744, 1986
Mansier P, Charlemagne D, Rossi B, Preteseille M, Swynghedauw B, Lelievre L: Isolation of impermeable inside-out vesicles from an enriched sarcolemma fraction of rat heart. J Biol Chem 258:6628–6635, 1983
Lukens FDW: Alloxan diabetes. Physiol Rev 28:304–330, 1948
Rerup CC: Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22:485–518, 1970
Bell RH, Hye RJ: Animal models of diabetes mellitus. Physiology and pathology. J Surg Res 35:433–460, 1983
Philipson KID: Methods for measuring sodium-calcium exchange in cardiac sarcolemmal vesicles. In: NS Dhalla (ed) Methods in Studying Cardiac Membranes. 1984, pp 147–155
Wharton DC, Tzagoloff A: Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 10:245–250, 1967
Aronson Jr NN, Touster O: Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 31:93–102, 1974
Hidalgo C, Parra C, Rioquelme G, Jaimovich E: Transverse tubules from frog skeletal muscle. Purification and properties of vesicles sealed with the inside-out orientation. Biochim Biophys Acta 855:79–88, 1986
Takahashi K, Kako KJ: The effect of a calcium channel antagonist, Nisoldipine, on the ischemia-induced change of canine sarcolemmal membrane. Basic Res Cardiol 78:326–337, 1983
Jones LR, Maddock SW, Besch Jr HR: Unmasking effect of alamethicin on the Na+K+ ATPase, beta adrenergic receptorcoupled adenylate cyclase and cAMP-depdendent protein kinase activities of cardiac sarcolemmal vesicles. J Biol Chem 255:9971–9980, 1980
Peterson CL: A simplification of the protein assay method of Lowry et al which is more generally applicable. Anal Biochem 83:346–356, 1977
Kato M, Kako KJ: Orientation of vesicles isolated from basolateral membranes of renal cortex. Mol Cell Biochem 78:9–16, 1987
Reeves JP, Bailey CA, Hale CC: Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem 261:4948–4955, 1986
Aust SD, Morehouse LA, Thomas CE: Role of metals in oxygen radical reactions. J Free Radicals Biol Med 1:3–25, 1985
Halliwell B, Gutteridge JMC: The importance of free radicals and catalytic: metal ions in human diseases. Mol Asp Med 8:89–193, 1985
Kako KJ: Membrane damage caused by lipid peroxidation in myocardial ischemia. Jikei Med J 32:609–639, 1985
Matsuoka T, Kato M, Kako KJ: Effects of hydrogen peroxide and hypochlorite on Na+K+ ATPase. (MS submitted) (Abstr) J Mol Cell Cardiol 19: Suppl IV-78, 1987
Weiss SJ, Klein R, Slivka A, Wei M: Chlorination of taurine by human neutrophils. Evidence for hyochlorous acid generation. J Clin Invest 70:598–607, 1982
Gupta MP, Makino N, Khatter K, Dhalla NS: Stimulation of Na+Ca2+ exchange in heart sarcolemma by insulin. Life Sci. 39:1077–1083, 1986
Goldstein S, Czapski G: The role and mechanism of metal ions and their complexes in enhancing damage in biological systems or in protecting these systems from the toxicity of O2. J Free Radicals Biol Med 2:3–11, 1986
Levine RL: Covalent modification of proteins by mixed function oxidation. Curr Top Cell Regul 27:305–316, 1985
Yip CC, Moule ML: Structure of the insulin receptor of rat adipocytes. Diabetes 32:760–767, 1983
Winterbourne CC: Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta 840:204–210, 1985
Kako KJ, Yanagishita T, Kato M, Kaminishi T, Matsuoka T: Mechanisms of oxidant-induced perturbation of calcium homeostasis in heart cells. In Medical and Biochemical Aspects of Free Radicals, Yoshikawa T (ed), Elsevier Publ. (in press) 1988
Rivett AJ, Roseman JE, Oliver CN, Levine RL, Stadtman ER: Covalent modification of proteins by mixed-function oxidation. Recognition by intracellular proteases. In Intracellular Protein Catabolism, ed EA Khairallah, JS Bond, JWC Bird, AR Liss, New York, 317–328, 1985
Davies KJA, Goldberg AL: Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem 262:8220–8226, 1987
Yamada S, Ikemoto N: Distinction of thiols involved in the specific reaction steps of the Ca2+ ATPase of the sarcoplasmic reticulum. J Biol Chem 253:6801–6807. 1978
Saito K, Yamashita T, Kubota I, Kawakita M: Reactive sulfhydryl groups of sarcoplasmic reticulum ATPase. J. Biochem. 101:365–376, 1987
Takahashi K, Kako KJ: The effect of myocardial ischemia and Nisoldipine pretreatment on the asymmetric distribution of phosphatidylethanolamine in a canine heart sarcolemmal preparation. Biochem Med Met Biol 35:308–321, 1986
Kako KJ: Isolation of sarcolemmal membrane, membrane orientation, and lipid asymmetry. Med Res Rev 7:507–522, 1987
Malaisse WJ: Alloxan toxicity to the pancreatic B-cell. A new hypothesis. Biochem Pharmacol 31:3527–3534, 1982
Black HE, Rosemblum TY. Chemically induced diabetes mellitus in the dog. Am J Pathol. 98:295–310, 1980
McEvoy RC, Hegre OD: Morphometric quantitation of the pancreatic insulin-, glucagon and somatostatin-positive cell populations in normal and alloxan diabetic rats. Diabetes 26:1140–1146, 1977
Steinman RM: Endocytosis and the recycling of plasma membrane. J Cell Biol 96:1–27, 1983
Shamoo AE, Ambudkar IS: Regulation of calcium transport in cardiac cells. Can J Physiol Pharmacol 62:9–22, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kato, M., Kako, K.J. Na+/Ca2+ exchange of isolated sarcolemmal membrane: effects of insulin, oxidants and insulin deficiency. Mol Cell Biochem 83, 15–25 (1988). https://doi.org/10.1007/BF00223194
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00223194