Abstract
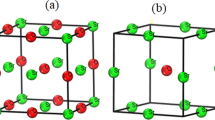
The isostructural lithium (Li2SiO3) and sodium (Na2SiO3) metasilicates have been investigated from room temperature up to the melting point by single-crystal Raman spectroscopy and energy-dispersive X-ray powder diffraction. The unit-cell parameters and Raman frequencies of Li2SiO3 vary regularly with temperature up to the melting point, which is consistent with the lack of premelting effects in calorimetric measurements. In contrast, Na2SiO3 undergoes a transition at about 850 K from orthorhombic Cmc 21 symmetry, to a lower symmetry (possibly Pmc 21), and shows near 1200 K changes in the Raman spectra that correlate well with the premelting effects as determined from calorimetry observations. In both compounds, a high alkali mobility likely sets in several hundreds of degrees below the melting point. Premelting in Na2SiO3 is associated with extensive deformation of the silicate chains as evidenced near the melting point by similarities in the Raman spectra of the crystalline and liquid phases.
Similar content being viewed by others
References
Brawer SA (1975) Theory of the vibrational spectra of some network and molecular glasses. Phys Rev B 11:3173–3194
Brawer SA, White WB (1975) Raman spectroscopic investigation of the structure of silicate glasses. I. The binary alkali silicates. J Chem Phys 63:2421–2432
Cameron M, Sueno S, Prewitt CT, Papike JJ (1973) High-temperature crystal chemistry of acmite, diopside, hedenbergite, jadeite, spodumene, and ureyite. Am Mineral 58: 594–618
Devarajan V, Shurvell HF (1977) Vibrational spectra and normal coordinate analysis of crystalline lithium metasilicate. Can J Chem 55:2559–2563
Dworkin AS, Bredig MA (1968) Diffuse transition and melting in fluorite and anti-fluorite type of compounds; heat content of potassium sulfide from 298 to 1260 °K. J Phys Chem 72: 1277–1281
Farnan I, Stebbins JF (1990) High-temperature 29Si NMR investigation of solid and molten silicates. J Am Chem Soc 112:32–39
Furukawa T, Fox KE, White WB (1981) Raman spectroscopic investigation of the structure of silicate glasses. III. Raman intensities and structural units in sodium silicate glasses. J Chem Phys 75:3226–3237
Gillet Ph, Riebet P, Guyot F, Fiquet G (1991) Anharmonicity and high-temperature thermodynamic properties of forsterite. J Geophys Res 96:11 805–11816
Gillet Ph, Fiquet G, Malézieux JM, Geiger C (1992) High-pressure and high-temperature Raman spectroscopy of end-member garnets: pyrope, grossular and andradite. Eur J Mineral 4:651–664
Hatton WE, Hildebrand DL, Sinke GC, Stull DR (1959) Thermodynamic properties of KOH, K2CO3, K2Si2O5, Li2SiO3, Li2Si2O5, and Ca(OH)2. Thermal Lab, Dow Chemical USA Rep. SSR 249–954
Hesse KF (1977) Refinement of the crystal structure of lithium polysilicate. Acta Cryst B 33:901–902
Kelley KK (1939) The specific heats at low temperatures of crystalline ortho, meta, and disilicates of sodium. J Am Chem Soc 61:471–473
Kieffer SW (1982) Thermodynamics and lattice vibrations of minerals: 5. Applications to phase equilibria, isotopic fractionation, and high-pressure thermodynamic properties. Rev Geophys Space Phys 20: 827–849
Konijnendijk WL, Stevels JM (1976) Raman scattering measurements of silicate glasses and compounds. J Non-Cryst Solids 21:447–453
Kracek FC (1930 a) The system sodium oxide-silica. J Phys Chem 34:1583–1598
Kracek FC (1930 b) The binary system Li2O-SiO2. J Phys Chem 34:2641–2650
Long DA (1977) Raman spectroscopy. McGraw Hill, New York, 277 pp
McDonald WS, Cruickshank DWJ (1967) A reinvestigation of the structure of sodium metasilicate. Acta Cryst 22:37–43
Matsuo T, Ohno H, Noda K, Konishi S, Yoshida H, Watanabe H (1983) Nuclear magnetic resonance investigation of lithium diffusion in Li2O, Li2SiO3 and LiAlO2. J Chem Soc Farad Trans 2, 79:1205–1216
Mysen BO (1988) Structure and properties of silicate melts. Elsevier, 354 pp
Mysen BO, Frantz JD (1992) Raman spectroscopy of silicate melts at magmatic temperatures: Na2O-Si2, and binary compositions in the temperature range 25–1475 °C. Chem Geol 96:321–332
Mysen BO, Frantz JD (1993) Structure and properties of alkali silicate melts at magmatic temperatures. Eur J Mineral 5:393–407
Mysen BO, Frantz JD (1994) Alkali silicate glass and melt structure in the temperature range 25–1651 °C at atmospheric pressure and implications for the mixing behavior of structural units. Contrib Min Petr 117:1–14
Mysen BO, Virgo D, Seifert FA (1982 a) The structure of silicate melts: implications for chemical and physical properties of natural magma. Rev Geophys 20:353–383
Mysen BO, Finger LW, Seifert FA, Virgo D (1982 b) Curve-fitting of Raman spectra of amorphous materials. Am Mineral 67:686–696
Naylor BF (1946) High-temperature heat contents of sodium metasilicate and sodium disilicate. J Am Chem Soc 67:466–467
Richet P, Fiquet G (1991) High-temperature heat capacity and premelting of minerals in the system MgO-CaO-Al2O3-SiO2. J Geophys Res 96:445–456
Richet P, Bottinga Y, Téqui C (1984) Heat capacity of sodium silicate liquids. J Am Ceram Soc 67:C6-C8
Richet P, Ingrin J, Mysen BO, Courtial P, Gillet Ph (1994) Premelting effects in minerals: an experimental study. Earth Planet Sci Lett 121:589–600
Stebbins JF, Carmichael ISE, Moret LK (1984) Heat capacities and entropies of silicate liquids and glasses. Contrib Min Petr 86:131–148
Téqui C, Grinspan P, Richet P (1992) Thermodynamic properties of alkali silicates: heat capacity of Li2SiO3 and lithium-bearing melts. J Am Ceram Soc 75:2601–2604
Ubbelhode AR (1978) The molten state of matter. John Wiley and Sons, New York
West AR (1977) Long-period superstructure in sodum-lithium metasilicates. Acta Cryst A 33:408–411
White WB (1974) Order-disorder effects. In: The infrared spectra of minerals. Farmer VC (ed.), Mineralogical Society, London, p 87–109
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richet, P., Mysen, B.O. & Andrault, D. Melting and premelting of silicates: Raman spectroscopy and X-ray diffraction of Li2SiO3 and Na2SiO3 . Phys Chem Minerals 23, 157–172 (1996). https://doi.org/10.1007/BF00220727
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00220727