Summary
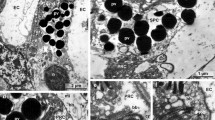
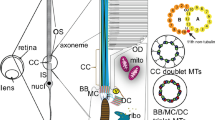
The eye of the tadpole larva of Amaroucium constellatum consists of 15–20 photoreceptor cells, a single pigment cup cell, and three lens cells. Each photoreceptor cell during its development gives rise apically to a neck process and basally to an axon, which is thought to extend to the cerebral ganglion. At the tip of the neck a sensory cilium differentiates to produce the photoreceptor membranes. This process begins as a balloon-shaped evagination of the cell membrane above the ciliary basal body. This evagination infolds to form the presumptive photosensitive lamellae which increase in number, length, and regularity as the eye matures. Microvilli also arise from the tip of this cell and intermingle with the ciliary membranes. The neck process itself elongates, narrows, and becomes filled with microtubules during development. The pigment cell, initially long, flat, and electron lucent, develops into a cupshape. Its cytoplasm becomes packed with pigment granules, forming an effective light shield. The development of the lens cells involves formation of the lens vesicles and elongation of the cells into the lumen of the pigment cup. Differentiation of ascidian photoreceptor cells is compared with that of the vertebrate rod and cone.
Similar content being viewed by others
References
Barnes, S. N.: The fine structure of the photoreceptor of the ascidian, Amaroucium constellatum. Biol. Bull. 137, 392 (1969)
Barnes, S. N.: Fine structure of the photoreceptor and cerebral ganglion of the tadpole larva of Amaroucium constellatun (Verrill) (Subphylum: Urochordata; Class: Acsidiacea). Z. Zellforsch. 117, 1–16 (1971)
Barnes, S. N.: Fine structure of the embryonic, larval, and metamorphic ascidian eye. Ph. D. thesis, University of Colorado (1972); also Dissert. Abstr. 33, 3429-B (1973)
Barnes, S. N., Gorman, A.L.F., McReynolds, J. S.: Fine structure and intracellular responses of photoreceptors of a pelagic tunicate, Salpa. Biol. Bull. 139, 414 (1970)
Bell, A. L., Barnes, S. N., Anderson, K. L.: A fixation technique for electron microscopy which provides uniformly good preservation of the tissues of a variety of marine invertebrates. Biol. Bull. 137, 393 (1969)
Bennett, H. S., Luft, J. H.: S-collidine as a basis for buffering fixatives. J. biophys. biochem. Cytol. 6, 113–114 (1959)
Brandenburger, J. L., Woollacott, R. M., Eakin, R. M.: Fine structure of eyespots in tornarian larvae (Phylum: Hemichordata). Z. Zellforsch. 142, 89–102 (1973)
Brightman, M. W-, Reese, T. S.: Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 40, 648–677 (1969)
Caley, D. W., Johnson, C., Liebelt, R. A.: The postnatal development of the retina in the normal and rodless CBA mouse: A light and electron microscopic study. Amer. J. Anat. 133, 179–212 (1972)
Carasso, N.: Étude au microscope électronique de la morphogenèse du segment externe des cellules visuelles chez la Pleurodèle. C. R. Acad. Sci. (Paris) 248, 3058–3060 (1959)
Cohen, A. I.: New evidence supporting the linkage to extracellular space of outer segment saccules of frog cones but not rods. J. Cell Biol. 37, 424–444 (1968)
Cohen, A. I.: An ultrastructural analysis of the photoreceptors of the squid and their synaptic connections. III. Photoreceptor terminations in the optic lobes. J. comp. Neurol. 147, 399–426 (1973)
Costello, D. P., Davidson, M. E., Eggers, A., Fox, M. H., Henley, C.: Methods for obtaining and handling marine eggs and embryos. Woods Hole, Massachusetts: Marine Biological Laboratory 1957
DeRobertis, E.: Morphogenesis of retinal rods. J. biophys. biochem. Cytol. 2, Suppl., 209–218 (1956)
DeRobertis, E., Lasansky, A.: Ultrastructure and chemical organization of photoreceptors. In: The structure of the eye (ed. G. K. Smelser), p. 29–49. New York: Academic Press 1961
Dilly, P. N.: Electron microscope observations of the receptors in the sensory vesicle of the ascidian tadpole. Nature (Lond.) 191, 786–787 (1961)
Dilly, P. N.: Studies on the receptors in the cerebral vesicle of the ascidian tadpole. 2. The ocellus. Quart. J. micr. Sci. 105, 13–20 (1964)
Dixon, J. S., Cronly-Dillon, J. R.: The fine structure of the developing retina in Xenopus laevis. J. Embryol. exp. Morph. 28, 659–666 (1972)
Dowling, J. E.: The organization of vertebrate visual receptors. In: Molecular organization and biological function (ed. J. M. Allen), p. 186–210. New York: Harper and Row 1967
Dowling, J. E.: Synaptic organization of the frog retina: an electron microscopic analysis comparing the retinas of frogs and primates. Proc. roy. Soc. B 170, 205–228 (1968)
Dudley, P. L.: The fine structure and development of the nauplius eye of the copepod Doropygus seclusus Illg. Cellule 68, 6–42 (1969)
Eakin, R. M.: Differentiation in the embryonic eye of Peripatus (Onychophora). 6th Int. Cong. E. M. 507–508 (1966)
Eakin, R. M.: Structure of invertebrate photoreceptors. In: Handbook of sensory physiology (ed. H. J. A. Dartnall), vol. VII/1, p. 625–684. Berlin-Heidelberg-New York: Springer 1972
Eakin, R. M., Brandenburger, J. L.: Differentiation in the eye of a pulmonate snail Helix aspersa. J. Ultrastruct. Res. 18, 391–421 (1967)
Eakin, R. M., Kuda, A.: Ultrastructure of sensory receptors in ascidian tadpoles. Z. Zellforsch. 112, 287–312 (1971)
Eakin, R. M., Kuda, A.: Glycogen in lens of tunicate tadpole (Chordata: Ascidiacea). J. exp. Zool. 180, 267–270 (1972)
Eakin, R. M., Westfall, J. A.: The development of photoreceptors in the stirnorgan of the treefrog, Hyla regilla. Embryologia (Nagoya) 6, 84–98 (1961)
Eakin, R. M., Westfall, J. A.: Fine structure of photoreceptors in Amphioxus. J. Ultrastruct. Res. 6, 531–539 (1962)
Eakin, R. M., Westfall, J. A.: Further observations on the fine structure of some invertebrate eyes. Z. Zellforsch. 62, 310–332 (1964)
Eguchi, E., Naka, K., Kuwabara, M.: The development of the rhabdom and the appearance of the electrical response in the insect eye. J. gen. Physiol. 46, 143–157 (1962)
Gorman, A.L.F., McReynolds, J. S., Barnes, S. N.: Photoreceptors in primitive chordates: Fine structure, hyperpolarizing potentials and evolution. Science 172, 1052–1054 (1971)
Grave, C.: Amaroucium pellucidum (Leidy), form constellatum (Verrill). I. The activities and reactions of the tadpole larva. J. exp. Zool. 30, 239–257 (1920)
Grave, C.: Amaroucium constellatum (Verrill). II. The structure and organization of the tadpole larva. J. Morph. 36, 71–101 (1921)
Grave, C., Riley, G.: Development of the sense organs of the larva of Botryllus schlosseri. J. Morph. 57, 185–211 (1935)
Gray, E. G.: Problems of interpreting the fine structure of vertebrate and invertebrate synapses. Int. Rev. gen. exp. Zool. 2, 139–170 (1966)
Hollenberg, M. J., Spira, A. W.: Human retinal development: ultrastructure of the outer retina. Amer. J. Anat. 137, 357–386 (1973)
Luft, J. H.: Improvements in epoxy embedding methods. J. biophys. biochem. Cytol. 9, 409–414 (1961)
Maser, M. D., Powell, T. E., Philpott, C. W.: Relationships among pH, osmolality, and concentration of fixative solutions. Stain Technol. 42, 175–182 (1967)
Mast, S. O.: Reactions to light in the ascidians Amaroucium constellatum and Amaroucium pellucidum with special reference to photic orientation. J. exp. Zool. 34, 149–187 (1921)
Minganti, A.: Ricerche istochimiche sulla localizzazione del territorio presuntivo degli organi sensoriali nella larva di Ascidie. Pubbl. Staz. Zool. Napoli 23, 52–57 (1951)
Minganti, A.: Inhibition of melanogenesis in Phallusia embryos (Ascidians). Acta Embryol. Morph. exp. (Palermo) 1, 37–47 (1957)
Moyer, F. H.: Development, structure, and function of the retinal pigmented epithelium. In: The retina: morphology, function and clinical characteristics (eds. B. R. Straatsma, M. O. Hall, R. A. Allen, F. Crescitelli), p. 1–30. Los Angeles: University of California Press 1969
Mund, M. L., Rodrigues, M. M., Fine, B. S.: Pigmentation in the developing eye. Amer. J. Ophthal. 73, 167–182 (1972)
Nakao, T.: On the fine structure of the Amphioxus photoreceptor. Tohoku J. exp. Med. 82, 349–369 (1964)
Nilsson, S.E.G.: Receptor cell outer segment development and ultrastructure of the disk membranes in the retina of the tadpole (Rana pipiens). J. Ultrastruct. Res. 11, 581–620 (1964)
Ochs, S.: Fast transport of materials in the mammalian nerve fibers. Science 176, 252–260 (1972)
Olney, J.: An E. M. study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest. Ophthal. 7, 250–268 (1968)
Palade, G. E.: A study of fixation for electron microscopy. J. exp. Med. 95, 285–298 (1952)
Reynolds, E.: The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963)
Richardson, K. C., Jarett, L., Finke, E. H.: Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 35, 313–323 (1960)
Röhlich, P.: Formation of the brush border by fusion of vesicles. Proc. 5th Int. Cong. E. M. 2, LL-5 (1962)
Sabatini, D. E., Bensch, K., Barrnett, R. J.: Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 17, 19–58 (1963)
Satir, P., Gilula, N. B.: The fine structure of membranes and intercellular communication in insects. Ann. Rev. Entomol. 18, 143–166 (1973)
Schwartz, W. J.: A septate-like contact in the rat retina. J. Neurocytol. 2, 85–89 (1973)
Scott, F. M. Sister: The developmental history of Amaruocium constellatum. I. Early embryonic development. Biol. Bull. 88, 126–138 (1945)
Scott, F. M. Sister: The developmental history of Amaroucium constellatum. II. Organogenesis of the larval action system. Biol. Bull. 91, 66–80 (1946)
Sjöstrand, F. S.: Electron microscopy of the retina. In: The structure of the eye (ed. G. K. Smelser), p 1–28. New York: Academic Press 1961
Sörensen, S. P. L.: Ergänzung zu der Abhandlung: Enzymstudien II: Über die Messung und die Bedeutung der Wasserstoffionenkonzentration bei enzymatischen Prozessen. Biochem. Z. 22, 352–356 (1909)
Takuyasu, K., Yamada, E.: The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments. J. biophys. biochem. Cytol. 6, 225–230 (1959)
Tilney, L. G., Porter, K. R.: Studies on the microtubules in Heliozoa. II. The effect of low temperature on the formation and maintenance of the axopodia. J. Cell Biol. 34, 327–343 (1967)
Waddington, C. H., Perry, M. M.: The ultra-structure of the developing eye of Drosophila. Proc. roy. Soc. B 153, 155–178 (1960)
Watson, M. L.: Staining of tissue sections for electron microscopy with heavy metals. J. biophys. biochem. Cytol. 4, 475–478 (1958)
Weidman, T. A., Kuwabara, T.: Postnatal development of the rat retina. An electron microscope study. Arch. Ophthal. 79, 470–484 (1968)
Weidman, T. A., Kuwabara, T.: Development of the rat retina. Invest. Ophthal. 8, 60–69 (1969)
Welsch, U.: Die Feinstruktur der Josephschen Zellen im Gehirn von Amphioxus. Z. Zellforsch. 86, 252–261 (1968)
Whittaker, J. R.: An analysis of melanogenesis in differentiating pigment cells of ascidian embryos. Develop. Biol. 14, 1–39 (1966)
Yamada, E., Ishikawa, T.: Some observations on the submicroscopic morphogenesis of the human retina. In: The structure of the eye (ed. J. Rohen), vol. II, p. 5–16 Stuttgart: F. K. Schattauer 1965
Young, R. W.: The renewal of photoreceptor cell outer segments. J. Cell Biol. 33, 61–72 (1967)
Author information
Authors and Affiliations
Additional information
This investigation was supported in part by NIH Grants number GM01981, RR06084, and EY00443.1 wish to acknowledge Dr. A. L. Bell for his assistance during this investigation, Drs. S. W. Smith, T. H. Goldsmith, and R. Wehner for critical reading of the manuscript, and Jeri Cohen for technical assistance.
Rights and permissions
About this article
Cite this article
Barnes, S.N. Fine structure of the photoreceptor of the ascidian tadpole during development. Cell Tissue Res. 155, 27–45 (1974). https://doi.org/10.1007/BF00220282
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00220282