Summary
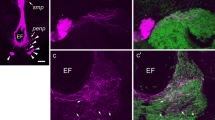
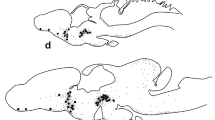
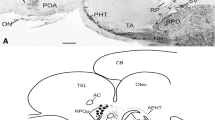
By use of a new antiserum, raised against synthetic pigment-dispersing hormone (PDH) from Uca pugilator, immunoreactive structures were studied at the light-microscopic level in the eyestalk ganglia of Carcinus maenas and Orconectes limosus. PDH-reactivity was mainly found in two types of neurons that were located between the medulla interna (MI) and the medulla terminalis (MT) in both species. Several additional perikarya were located in the distal part of the MI in O. limosus. In C. maenas, two to three PDH-positive perikarya were found in the region of the X-organ (XO) in the MT. Processes from single and clustered cells could be traced into all medullae of the eyestalk. Axons from the immunoreactive perikarya running between MI and MT form a larger tract that traverses the MT. Fibers from this tract give rise to extensive arborizations and plexuses throughout the proximal MT. A plexus containing very fine fibers is located at the surface of the MT in a position distal to the XO-area of C. maenas only. The proximal plexus also receives PDH-positive fibers through the optic nerve. PDH-perikarya in the cerebral ganglion may also project into the more distal regions of the eyestalk. Distal projections of the perikarya between the MI and MT consist of several branches. Most of these are directed toward the MI and ME (medulla externa) wherein they form highly organized, layered plexuses. One branch was traced into the principal neurohemal organ, the sinus gland (SG). In the SG, the tract gives off arborizations and neurosecretory terminals. It then proceeds in a proximal direction out of the SG, adjacent to the MT. Its further course could not be elucidated. The lamina ganglionaris (LG) receives PDH-fibers from the ME and fine processes from small perikarya located in close association with the LG in the distal part of the first optic chiasma. The architecture of PDH-positive elements was similar in both C. maenas and O. limosus. The distribution of these structures suggests that PDH is not only a neurohormone but may, in addition, have a role as a neurotransmitter or modulator. Immunostaining of successive sections with an FMRF-amide antiserum revealed co-localization of FMRFamideand PDH-immunoreactivities in most, but not all PDH-containing perikarya and fibers. The axonal branch leading to the SG and the SG proper were devoid of FMRFamide immunoreactivity.
Similar content being viewed by others
References
Boer HH, Schot LPC, Roubos EW, ter Maat A, Lodder JC, Reichelt D (1979) ACTH-like immunoreactivity in two electrotonically coupled giant neurons in the pond snail Lymnaea stagnalis. Cell Tissue Res 202:231–240
Dircksen H, Zahnow CA, Keller R, Gaus G, Rao KR, Riehm JP (1987) The ultrastructure of nerve endings containing pigment dispersing hormone (PDH) in crustacean sinus glands identified by an antiserum against a synthetic PDH. Cell Tissue Res 250:377–387
Fernlund P (1976) Structure of a light-adapting hormone from the shrimp, Pandalm borealis. Biochem biophys Acta 439:17–25
Fingerman M, Couch EF (1967) Differentiation of chromatophorotropins from the prawn, Palaemonetes vulgaris, and the fiddler crab, Uca pugilator. J Exp Zool 165:183–194
Fingerman M, Bartell CK, Krasnow RA (1971) Comparison of chromatophorotropins from the horseshoe crab Limulus polyphemus, and the fiddler crab, Uca pugilator. Biol Bulletin 140:376–388
Gorgels-Kallen J, Van Herp F, Leuven RSEW (1982) A comparative immunocytochemical investigation of the crustacean hyperglycemic hormone (CHH) in the eyestalk of some decapod Crustacea. J Morphol 174:161–168
Greenberg MJ, Price DA (1983) Invertebrate neuropeptides: Native and naturalized. Ann Rev Physiol 45:271–288
Hanström B (1924) Untersuchungen über das Gehirn, insbesondere die Sehganglien der Crustaceen. Ark Zool 16:1–119
Hooper SL, Marder E (1984) Modulation of a central pattern generator by two neuropeptides, proctolin and FMRFamide. Brain Res 305:186–191
Jacobs AAC, Van Herp F (1984) Immunocytochemical localization of a substance in the eyestalk of the prawn, Palaemon serratus, reactive with an anti-FMRFamide rabbit serum. Cell Tissue Res 235:601–605
Jaros PP, Keller R (1979) Immunocytochemical identification of hyperglycemic hormone-producing cells in the eyestalk of Carcinus maenas. Cell Tissue Res 204:379–385
Keller R (1983) Biochemistry and specificity of the neurohormonal hormones in Crustacea. In: Gupta AP (ed) Neurohemal Organs of Arthropods, Charles C. Thomas pp 118–148
Keller R, Kegel G (1984) Studies on crustacean neuropeptides by use of high performance liquid chromatography. In: Hoffmann JA, Prochet M (eds) Biosynthesis, metabolism and mode of action of invertebrate hormones. Springer, Berlin Heidelberg New York pp 145–154
Keller R, Jaros PP, Kegel G (1985a) Crustacean hyperglycemic neuropeptides. Amer Zool 25:207–221
Keller R, Dircksen H, Mangerich S, Jaros PP (1985b) Hyperglycemic hormone and other neuropeptides in crustacean neurosecretory structures. In: Kobayashi H, Bern HA, Urano A (eds) Neurosecretion and the biology of neuropeptides, Japan Sci Soc Press Tokyo/Springer, Berlin Heidelberg New York 60–69
Kleinholz H (1970) A progress report on the separation and purification of crustacean neurosecretory pigmentary-effector hormones. Gen Comp Endocrinol 14:578–588
Kleinholz EH (1975) Purified hormones from the crustacean eyestalk and their physiological specificity. Nature 258:256–257
Kleinholz EH, Keller R (1979) Endocrine regulation in Crustacea. In: Barrington EJW (ed) Hormones and evolution, Academic Press NY pp 158–213
Kleinholz LH, Rao KR, Riehm JP, Tarr GE, Johnson E, Norton S (1986) Isolation and sequence analysis of a pigment-dispersing hormone from eyestalks of the crab, Cancer magister. Biol Bull 170:135–143
Mancillas JR, McGinty JF, Selverston AI, Karten H, Bloom F (1981) Immunocytochemical localization of enkephalin and substance P in retina and eyestalk neurones of lobster. Nature 293:576–578
Mangerich S, Keller R, Dircksen H (1986) Immunocytochemical identification of structures containing putative red pigment-concentrating hormone in two species of decapod crustaceans. Cell Tissue Res 245:377–386
Pezalla PD, Dores RM, Herman WS (1978) Separation and partial purification of central nervous system peptides from Limulus polyphemus with hyperglycemic and chromatophorotropic activity in crustaceans. Biol Bull 154:148–157
Price DA, Greenberg MJ (1977) Structure of a molluscan cardioexcitatory neuropeptide. Science 199:670–671
Rao KR (1985) Pigmentary effectors. In: Bliss DE, Mantel EH (eds) The biology of Crustacea Vol 9, Academic Press, pp 395–462
Rao KR, Riehm JP, Zahnow CA, Kleinholz EH, Tarr GE, Johnson E, Norton S, Eandau M, Semmes OJ, Sattelberg RM, Jorenby WH, Hintz MF (1985) Characterization of a pigment-dispersing hormone in eyestalks of the fiddler crab, Uca pugilator. Proc Nat Sci USA 82:5319–5323
Riehm JP, Rao KR (1982) Structure-activity relationship of a pigment dispersing crustacean neurohormone. Peptides 3:643–647
Riehm JP, Rao KR, Semmes OJ, Jorenby WH, Hintz MF, Zahnow CA (1985) C-terminal deletion analogs of a crustacean pigment-dispersing hormone. Peptides 6:1051–1056
Shibley GA (1968) Eyestalk function in chromatophore control in a crab, Cancer magister. Physiol Zool 41:268–279
Sternberger EA (1974) Immunocytochemistry. Prentice Hall Inc, Englewood Cliffs, New Jersey
Van Deijnen JE, Vek F, Van Herp F (1985) An immunocytochemical study of the optic ganglia of the crayfish Astacus leptodactylus with antisera against biologically active peptides of vertebrates and invertebrates. Cell Tissue Res 240:175–183
Van Herp F, Van Buggenum HJM (1979) Immunocytochemical localization of hyperglycemic hormone (HGH) in the neurosecretory system of the eyestalk of the crayfish Astacus leptodactylus. Experientia 35:1527–1529
Webster SG, Keller R (1987) Physiology and biochemistry of crustacean neurohormonal peptides. In: Thorndyke M, Goldsworthy G (eds) Neurohormones in invertebrates, Cambridge University Press (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mangerich, S., Keller, R., Dircksen, H. et al. Immunocytochemical localization of pigment-dispersing hormone (PDH) and its coexistence with FMRFamide-immunoreactive material in the eyestalks of the decapod crustaceans Carcinus maenas and Orconectes limosus . Cell Tissue Res. 250, 365–375 (1987). https://doi.org/10.1007/BF00219081
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00219081