Abstract
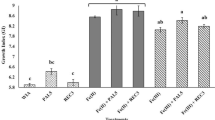
The effects of catechol, vanillic, caffeic (CAF), 2-hydroxyphenylacetic, 4-hydroxy- and 3,4-dihydroxybenzoic (3,4-DHBA) acids on the growth of a common rice rhizosphere inhabitant, Azospirillum lipoferum were studied. Two strains of this nonfermenting nitrogen-fixing bacterium were used: a motile strain (4B), and a nonmotile strain (4T). Under atmospheric conditions (pO2 = 21 kPa), the growth of strain 4T was inhibited by catechol (0.1 mm) only. None of these compounds affected the growth of strain 413. Under 5 kPa O2, no effect was observed on strain 413, whereas three of the six tested phenolics stimulated the growth of strain 4T; maximum effects were observed for 3,4-DHBA and CAF. As revealed by TLC and HPLC, under low oxygen, more new lipophilic compounds were formed from CAF by strain 4T, differing from CAF autooxydation products and from the products obtained under 21 kPa O2. It was hypothesized that strain 4T had the ability to use an oxidized derivative of CAF as a terminal electron acceptor. This hypothesis was tested in experiments under nitrogen-fixing conditions, in the absence of oxygen, and in the presence of N2O as a reoxidizing agent for CAF. Acetylene was used both as a substrate to measure nitrogenase activity (ARA) and to inhibit the biological transfer of electrons to N2O. The addition of CAF in the presence of N2O had the same effect on ARA rates as an addition of oxygen. It is concluded that the strain 4T of Azospirillum lipoferum is able to sustain some of its activities (e.g., N2 fixation) using phenolics as alternative electron acceptors under low oxygen conditions.
Similar content being viewed by others
References
Atlas RM, Bartha R (1981) Microbial ecology: fundamentals and applications. Addison-Wesley Pub., Reading, Mass.
Bally R, Thomas-Bauzon D, Heulin T, Balandreau J, Richard C, De Ley J (1983) Determination of the most frequent N2-fixing bacteria in a rice rhizosphere. Can J Microbiol 29:881–887
Barkovskii AL (1993) Biodestructive potential of symbiotic and of associative microorganisms to aromatic and haloaromatic compounds. In: Edyvean RG (ed) Effluent treatment and waste minimisation. (Chem Symp. ser., 132) Chameleon Press, London, pp 157–169
Barkovskii AL, Shub GM (1986) An Acinetobacter calcoaceticus strain utilizing various aromatic compounds and carrying a plasmid for resorcinol degradation. Mikrobiologija 55:237–240
Bassam BJ, Djordjevic MA, Redmond JW, Batley M, Rolfe BG (1988) Identification of a nodD-dependent locus in the Rhizobium strain NG234 activated by phenolic factor secreted by soybeans and other legumes. Mol Plant-Microbe Interact 1:161–168
Blum U, Wentworth TR, Klein K, Worsham AD, King LD, Geric TM, Lyu SW (1991) Phenolic acid content of soil from wheat no-till, wheat conventional-till, and fallow conventional-till soybean cropping system. J Chem Ecol 17:1045–1068
Burns RC, Hardy RWF (1975) Nitrogen fixation in bacteria and higher plants. Springer-Verlag, Berlin
Crane FL, Barr R (1985) Chemical structure and properties of coenzyme Q and related compounds. In: Lenaz G (ed) Coenzyme Q. Biochemistry, bioenergetics, and clinical applications of ubiquinone. Wiley & Sons, New York, pp 1–39
Dagley S (1967) The microbial metabolism of phenolics. In: McLaren AD, Peterson GH (eds) Soil biochemistry. Marcel Dekker, Inc., New York, pp 287–317
Deiana S, Gessa C, Manunza B, Marchetti M, Usai M (1992) Mechanism and stoichiometry of the redox reaction between iron (III) and caffeic acid. Plant Soil 145:287–294
Einarsdottir GH, Stankovich MT, Tu S-C (1988) Studies of electron-transfer properties of salicylate hydroxylase from Pseudomonas cepacia and effects of salicylate and benzoate binding. Biochemistry 27:3277–3285
Flaig W, Beutelspacher H, Rietz E (1975) Chemical composition and physical properties of humic substances. In: Gieseking JE (ed) Soil components: v.l. Organic components. Springer-Verlag, Berlin, pp 1–211
Flessa A, Fischer WR (1992) Plant-induced changes in the redox potential of rice rhizospheres. Plant Soil 143:55–60
Gajendiran N, Mahadevan A (1990) Plasmid-born catechol dissimilation in Rhizobium sp. FEMS Microbiol Ecol 73:125–130
Gasser F, Biville F, Turline G (1991) Un nouveau cofacteur d'oxydoreduction, la pyrroloquinoline quinone. Ann Inst Pasteur/Actualités 2:139–149
Givaudan A, Effosse A, Bally R (1991) Melanin production by Azospirillum lipoferum strains. In: Polsinelli M, Materassi R, Vincenzini M (eds) Nitogen fixation. (Developments in plant and soil sciences) Kluwer Academic, Dordrecht, pp 311–312
Givaudan A, Effosse A, Faure D, Potier P, Bouillant ML, Bally R (1993) Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: evidence of a laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol Let 108:205–210
Haider K, Martin JP (1975) Decomposition of specifically carbon-14 labelled benzoic acid and cinnamic acid derivatives in soil. Soil Sci Soc Am Proc 39:657–662
Hardwood CS, Rivelli M, Orston LN (1984) Aromatic acids are chemoattractants for Pseudomonas putida. J Bacteriol 169:622–628
Harris RF (1982) Energetics of nitrogen transformations. In: Stevenson FJ (ed) Nitrogen in agricultural soils. (Agronomy series 22) ASA, CSA, and SSSA Pub, Madison, Wisconsin, pp 833–890
Hartley RD, Whitehead DC (1985) Phenolic acids in soils and their influence on plant growth and soil microbial processes. In: Vaughan D, Malcolm RE (eds) Soil organic matter and biological activity. Martinus Nijhoff/DR W. Junk, Dordrecht, Germany, pp 109–149
Hartmann A, Fusseder A, Klingmuller W (1983) Mutants of Azospirillum affected in nitrogen fixation and auxin production. In: Klingmüller W (ed) Azospirillum. II. Genetics, physiology, ecology. (Experientia Suppl. 48) Birhäuser Verlag, Basel, pp 78–88
Havelka UD, Boyle MG, Hardy RWF (1982) Biological nitrogen fixation. In: Stevenson FJ (ed) Nitrogen in agricultural soils. (Agronomy series 22) ASA, CSA, and SSSA Pub, Madison, Wisconsin, pp 365–422
Heulin T, Weinhard P, Balandreau J (1983) Motility changes in Azospirillum lipoferum. In: Klingmuller W (ed) Azospirillum. II. Genetics, physiology, ecology. (Experientia Suppl 48) Birkhäuser Verlag, Basel, pp 89–94
Kape R, Parniske M, Werner D (1991) Chemotaxis and nod gene activity of Bradyrhizobium japonicum in response to hydroxycinnamic acids and isoflavonoids. Appl Environ Microbiol 57:316–319
Karasevitch Yu N (1982) The foundation of selection for microorganisms utilizing synthetic organic compounds. Mir, Moscow
Korzhenevitch VI, Ignatov OV, Mironov AD, Krivopalov VV, Barkovskii AL (1991) Activity of bacterial strains destructors of aromatic compounds entrapped in agar gel beads. Prikladnaja Biokhim Mikrobiol 27:365–369
Krotzky A, Berggold R, Jaeger D, Dart PJ, Werner D (1983) Enhancement of aerobic nitrogenase activity (acetylene reduction assay) by phenol in soils and the rhizosphere of cereals. Z Pflanzenernaeher Bodenkd 146:634–642
Kuiters AT, Dennemann CAJ (1987) Water soluble phenolic substances in soils under several coniferous and deciduous tree species. Soil Biol Biochem 19:765–769
Kumada K (1987) Chemistry of soil organic matter. Elsevier, Amsterdam
Lehmann RG, Chang HH (1988) Reactivity of phenolic acids in soil and formation of oxidation products. Soil Sci Soc Am J 52:1304–1309
Lehmann RG, Cheng HH, Harsh JB (1987) Oxidation of phenolic acids by soil iron and manganese oxides. Soil Sci Soc Am J 51:352–356
Le Strange KK, Bender GL, Djordjevic MA, Rolfe BG, Redmond JW (1990) The Rhizobium strain NGR234 nodD1 gene product responds to activation by the simple phenolic compounds vanillin and isovanillin present in wheat seedling extracts. Mol Plant-Microbe Interact 3–4:214–220
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10
Lynn DG, Chang M (1990) Phenolic signals in cohabitation: implications for plant development. Annu Rev Plant Physiol Plant Mol Biol 41:497–526
Pflug W, Ziechmann W (1981) Inhibition of malate dehydrogenase by humic acids. Soil Biol Biochem 13:239–299
Schnitzer M (1978) Humic substances: chemistry and reactions. In: Schnitzer M, Khan SU (eds) Soil organic matter. Elsevier, Amsterdam, pp 1–64
Spaling GP, Ord BG, Vaughan D (1981) Changes in microbial biomass and activity in soils amended with phenolic compounds. Soil Biol Biochem 13:455–460
Swallow AJ (1982) Physical chemistry of semiquinones. In: Trumpower BL (ed) Function of quinones in energy conserving systems. Academic Press, London, pp 59–73
Thomas-Bauzon D, Weinhard P, Villecourt P, Balandreau J (1982) The spermosphere model. 1. Its use in growing, counting, and isolating nitrogen-fixing bacteria from the rizosphere of rice. Can J Microbiol 28:922–928
Ueckert J, Hurek T, Fendrik I, Niemann EG (1990) Radial gas diffusion from roots of rice (Oryza sativa L.) and Kallar grass (Leptochloa fuska L. Kunth. ), and effect of inoculation with Azospirillum brasifense Cd. Plant Soil 122:59–65
Vançura V, Kunc F (1988) Soil microbial associations: control of structures and functions. Elsevier, Amsterdam
von Ziechmann W (1972) deÜber die elektronen-Donator-und-Acceptor-Eingenschaften von Huminstoffen. Geoderma 8:111–131
von Ziechmann W (1977) MolekülKomplexe bei Huminstoffen durch e-Donator- und e-AcceptorStrukturen. Z Pflanzenernaehr Bodenkd 140:133–150
Werner D, Krotzky A, Berggold R, Thierfelder H, Preiss M (1982) Enhancement of specific nitrogenase activity in Azospirillum brasilense and Klebsiella pneumoniae, inhibition in Rhizobium japonicum under air by phenol. Arch Microbiol 132:51–56
Whitehead DC, Dibb H, Hartley RD (1983) Bound phenolic compounds in water extracts of soils, plant roots, and leaf litter. Soil Biol Biochem 15:133–136
Yoshinari T, Hynes R, Knowles R (1977) Acetylene inhibition of nitrous oxide reduction and measurement of denitrification and nitrogen fixation in soil. Soil Biol Biochem 9:177–183
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barkovskii, A., Bouillant, M.L., Monrozier, L.J. et al. Azospirillum strains use phenolic compounds as intermediates for electron transfer under oxygen-limiting conditions. Microb Ecol 29, 99–114 (1995). https://doi.org/10.1007/BF00217426
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00217426