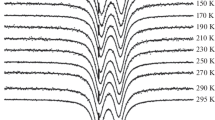
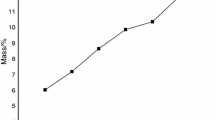
Abstract
The lepidocrocite (γ-FeOOH) to maghemite (γ-Fe2O3), and the maghemite to hematite (α-Fe2O3) transition temperatures have been monitored by TGA and DSC measurements for four initial γ-FeOOH samples with different particle sizes. The transition temperature of γ-FeOOH to γ-Fe2O3 and the size of the resulting particles were not affected by the particle size of the parent lepidocrocite. In contrast, the γ-Fe2O3 to γ-Fe2O3 transition temperature seems to depend on the amount of excess water molecules present in the parent lepidocrocite. Thirteen products obtained by heating for one hour at selected temperatures, were considered. Powder X-ray diffraction was used to qualify their composition and to determine their mean crystallite diameters. Transmission electron micrographs revealed the particle morphology. The Mössbauer spectra at 80 K and room temperature of the mixed and pure decomposition products generally had to be analyzed with a distribution of hyperfine fields and, where appropriate, with an additional quadrupole-splitting distribution. The Mössbauer spectra at variable temperature between 4.2 and 400 K of two single-phase γ-Fe2O3 samples with extremely small particles show the effect of superparamagnetism over a very broad temperature range. Only at the lowest temperatures (T⩽55 K), two distributed components were resolved from the magnetically split spectra. In the external-field spectra the ΔmI=0 transitions have not vanished. This effect is an intrinsic property of the maghemite particles, indicating a strong spin canting with respect to the applied-field direction. The spectra are successfully reproduced using a bidimensional-distribution approach in which both the canting angle and the magnetic hyperfine field vary within certain intervals. The observed distributions are ascribed to the defect structure of the maghemites (unordered vacancy distribution on B-sites, large surface-to-bulk ratio, presence of OH- groups). An important new finding is the correlation between the magnitude of the hyperfine field and the average canting angle for A-site ferric ions, whereas the B-site spins show a more uniform canting. The Mössbauer parameters of the two hematite samples with MCD104 values of respectively 61.0 and 26.5 nm display a temperature variation which is very similar to that of small-particle hematites obtained from thermal decomposition of goethite. However, for a given MCD the Morin transition temperature for the latter samples is about 30 K lower. This has tentatively been ascribed to the different mechanisms of formation, presumably resulting in slightly larger lattice parameters for the hematite particles formed from goethite, thus shifting the Morin transition to lower temperatures.
Similar content being viewed by others
References
Amin N, Arajs S (1987) Morin temperature of annealed submicronic α-Fe2O3 particles. Phys Rev B 35:4810–4811
Annersten H, Hafner SS (1973) Vacancy distribution in synthetic spinels of the series Fe3O4 — γ-Fe2O3. Z Kristallogr 137:321–340
Batis-Landoulsi H, Vergnon P (1983) Magnetic moment of γ-Fe2O3 microcrystals: morphological and size effect. J Mater Sci 18:3399–3403
Berkowitz AE, Schuele WJ, Flanders PJ (1968) Influence of crystallite size on the magnetic properties of acicular γ-Fe2O3 particles. J Appl Phys 39:1261–1263
Bernal JD, Dasgupta DR, Mackay AL (1957) Oriented transformations in iron oxides and hydroxides. Nature 180:645–647
Brett ME, Graham MJ (1986) An electron back-scattering Mössbauer spectroscopy study of thin magnetite films. J Magn Magn Mater 60:175–181
Bruzzone CL, Ingalls R (1983) Mössbauer-effect study of the Morin transition and atomic positions in hematite under pressure. Phys Rev B 28:2430–2440
Coey JMD (1971) Noncollinear spin arrangement in ultrafine ferrimagnetic crystallites. Phys Rev Lett 27:1140–1142
Coey JMD (1987) Noncollinear spin structures. Can J Phys 65:1210–1232
Coey JMD, Khalafalla D (1972) Superparamagnetic γ-Fe2O3. Phys Status Solidi (a) 11:229–241
de Bakker PMA, De Grave E, Persoons RM, Bowen LH, Vandenberghe RE (1990 a) An improved, two-parameter distribution method for the description of the Mössbauer spectra of magnetic small particles in an applied field. J Phys: Meas Sci Technol 1:954–964
de Bakker PMA, De Grave E, Vandenberghe RE, Bowen LH (1990b) Mössbauer study of small-particle maghemite. Hyperfine Interactions 54:493–498
De Grave E, Vandenberghe RE (1990) Mössbauer study of the spin structure in natural hematites. Phys Chem Minerals 17:344–352
De Grave E, Chambaere D, Bowen LH (1983) Nature of the Morin transition in Al-substituted hematite. J Magn Magn Mater 30:349–354
De Grave E, Persoons RM, Chambaere DG, Vandenberghe RE, Bowen LH (1986) An 57Fe Mössbauer effect study of poorly crystalline γ-FeOOH. Phys Chem Minerals 13:61–67
De Grave E, Bowen LH, Vochten R, Vandenberghe RE (1988) The effect of crystallinity and Al substitution on the magnetic structure and Morin transition in hematite. J Magn Magn Mater 72:141–151
Farell DM (1972) A study of the infrared absorption in the oxidation of magnetite to maghemite and hematite. Mines Branch Inv. Rept. 72–18: Dept. Energy Mines Res., Ottawa, Ontario, Canada, pp 44
Feitknecht W, Mannweiler U (1967) Der Mechanismus der Umwandlung von γ- zu α-Eisensesquioxid. Helv Chim Acta 50:570–581
Feitknecht W, Michaelis W (1962) Über die Hydrolyse von Eisen(III)-perchlorat-Lösungen. Helv Chim Acta 45:212–224
Goss CJ (1988) Saturation magnetization, coercitivity and lattice parameter changes in the system Fe3O4 — γ-Fe2O3 and their relationship to structure. Phys Chem Minerals 16:164–171
Greaves C (1983) A powder neutron diffraction investigation of vacancy ordering and covalency in γ-Fe2O3. J Solid State Chem 49:325–333
Haneda K, Morrish AH (1977a) Vacancy ordering in γ-Fe2O3 small particles. Solid State Commun 22:779–782
Haneda K, Morrish AH (1977b) On the hyperfine field of γ-Fe2O3 small particles. Phys Lett 64A:259–262
Jing J, Zhao F, Yang X, Gonser U (1990) Magnetic relaxation in nanocrystalline iron-oxides. Hyperfine Interactions 54:571–576
Klug HP, Alexander LE (1974) X-ray diffraction procedures for polycristalline and amorphous materials. Wiley, New York, pp 687–690
Koch CJW, Madsen BM, Mørup S (1986) Decoupling of magnetically interacting crystallites of goethite. Hyperfine Interactions 28:549–552
Kubo R (1952) The spin-wave theory of antiferromagnetics. Phys Rev 87:568–580
Lewis GK Jr, Drickamer HG (1966) Effect of high pressure on the lattice parameters of Cr2O3 and α-Fe2O3. J Chem Phys 45:224–226
Morrish AH, Clark PE (1974) Non-collinearity as a size effect in micropowders of γ-Fe2O3. Proc Int Conf Magn, Vol II, Moscow-USSR, Publishing House “Nauka”, pp 180–184
Morrish AH, Haneda K, Schurer PJ (1976) Surface magnetic stucture of small γ-Fe2O3 particles. J Phys Colloq C6 37:301–305
Mørup S (1983) Magnetic hyperfme splitting in Mössbauer spectra of microcrystals. J Magn Magn Mater 37:39–50
Mørup S, Topsøe H (1976) Mössbauer studies of thermal excitations in magnetically ordered microcrystals. Appl Phys 11:63–66
Muench GJ, Arajs S, Matijevic E (1985) The Morin transition in small α-Fe2O3 particles. Phys Status Solidi (a) 92:187–192
Nininger RC Jr, Schroeer D (1978) Mössbauer studies of the Morin transition in bulk and microcrystalline α-Fe2O3. J Phys Chem Solids 39:137–144
Ochi A, Watanabe K, Kiyama M, Shinjo T, Bando Y, Takada T (1981) Surface magnetic properties of γ-Fe2O3 by 57Fe Mössbauer emission spectroscopy. J Phys Soc Jpn 50:2777–2778
Okada T, Sekizawa H, Ambe F, Ambe S, Yamadaya T (1983) Magnetic and Mössbauer studies of Co adsorbed γ-Fe2O3. J Magn Magn Mater 31:903–904
Picone PJ, Haneda K, Morrish AH (1982) Dynamic and magnetic excitations in ultrafine particles. J Phys C: Solid State Phys 15:317–327
Pollard RJ (1990) The spin-canting anomaly in ferrimagnetic particles. J Phys: Condens Matter 2:983–991
Pollard RJ, Morrish AH (1987) High-field magnetism in non-polar γ-Fe2O3 recording particles. IEEE Trans Magn MAG-23:42–44
Ramdani A, Steinmetz J, Gleitzer C, Coey JMD, Friedt JM (1987) Perturbation de l'échange électronique rapide par les lacunes cationique dans Fe3-xO4 (x⩽0.09). J Phys Chem Solids: 217–228
Rendon JL, Cornejo J, De Arambarri P, Serna CJ (1983) Pore structure of thermally treated goethite (α-FeOOH). J Colloid Interface Sci 92:508–516
Sidhu PS (1988) Transformation of trace element-substituted maghemite to hematite. Clays Clay Minerals 36:31–38
Subrt J, Hanousek F, Zapletal V, Lipka J, Hucl M (1981) Dehydration of synthetic lepidocrocite (γ-FeOOH). J Thermal Anal 20:61–69
Takada T, Kiyama M, Shimizu S (1964) Morphological and crystallographical studies on the oriented transformation in γ-FeOOH and its decomposed oxides. Bull Inst Chem Res, Kyoto Univ 42:505–510
Tamura I, Hayashi M (1988) Magnetic interactions among closely packed γ-Fe2O3 microcrystals studied by Mössbauer spectroscopy. J Magn Magn Mater 72:285–294
Vandenberghe RE, De Grave E (1989) In: Long GJ, Grandjean F (eds) Mössbauer Spectroscopy Applied to Inorganic Chemistry, Vol 3. Plenum Press, New York, pp 59–182
Vaughan RW, Drickamer HG (1967) High-pressure Mössbauer studies on α-Fe2O3, FeTiO3 and FeO. J Chem Phys 47:1530–1536
Verbeeck AE, De Grave E, Vandenberghe RE (1986) The effect of the particle morphology on the Mössbauer effect in α-Fe2O3. Hyperfine Interactions 28:639–642
Watari F, Van Eanduyt J, Delavignette P, Amelinckx S (1979) Electron microscopic study of dehydration transformations. I. Twin formation and mosaic structure in hematite derived from goethite. J Solid State Chem 29:137–150
Watari F, Van Landuyt J, Delavignette P, Amelinckx S, Igita N (1982) X-ray peak broadening as a result of twin formation in some oxides derived by dehydration. Phys Status Solidi (a) 73:215–224
Wivel C, Mørup S (1981) Improved computational procedure for evaluation of overlapping hyperfine parameter distributions in Mössbauer spectra. J Phys E: Sci Instrum 14:605–610
Wolska E, Baszynski J (1986) Prereactional transformations in the topotatic conversion γ-FeOOH→γ-Fe2O3. Phys Status Solidi (a) 95:87–92
Author information
Authors and Affiliations
Additional information
Senior Research Associate, National Fund for Scientific Research (Belgium)
Rights and permissions
About this article
Cite this article
de Bakker, P.M.A., De Grave, E., Vandenberghe, R.E. et al. Mössbauer study of the thermal decomposition of lepidocrocite and characterization of the decomposition products. Phys Chem Minerals 18, 131–143 (1991). https://doi.org/10.1007/BF00216606
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216606