Abstract
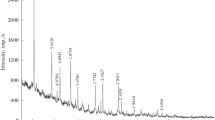
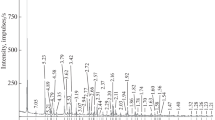
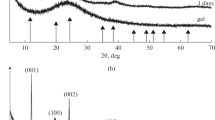
The thermal behavior of Tunisian phosphorite was investigated with X-ray powder diffraction (XRD), Fourier transform infrared (FT-IR), and Raman spectroscopies and DTA-TG measurements. The XRD patterns showed that the chief mineral constituents of calcined samples are calcium oxide and fluorapatite, while those in the raw phosphorite are calcite, dolomite, fluorapatite, and carbonate-fluorapatite. Physicochemical transformations result in the oxidation of organic matter, disappearance of calcite and dolomite crystalline phases, and partial dissociation of structural carbonates. The FT-IR and Raman spectra showed modifications of some bands; a decrease in the intensity of the v2 and v3 vibrations of carbonate groups and the appearance of new bands at 520 and 926 cm−1 after calcination of phosphorites at 800 °C. These bands were assigned to isomorphous substitutions of PO4 3− in apatite by SiO4 4−. Heat treatment alters the qualitative composition of the mineral as a result; the solubility of apatite in dilute citric acid was decreased.










Similar content being viewed by others
References
Kolevaa V, Petkova V. IR spectroscopic study of high energy activated Tunisian phosphorite. J Vibra Spec. 2012;58:125–32.
Da Silva EF, Mlayah A, Gomes C, Noronha F, Cristina Sequeira C, Estevesd V, Marquesd ARF. Heavy elements in the phosphorite from Kalaat Khasba mine (North-westernTunisia): potential implications on the environment and human health. J Hazard Mater. 2010;182:232–45.
Khaddor M, Ziyad M, Joffre J, Amblés A. Pyrolysis and characterization of the kerogen from the Morrocan Youssoufia rock phosphate. Chem Geol. 2002;186:17–30.
Abouzeid A-ZM. Physical and thermal treatment of phosphate ores—an overview. Int J Miner Process. 2008;85:59–84.
Blazy P, Jdid E A. Décarbonisation des phosphates sédimentaires par calcination dynamique. C. R. Acad. Sci. Paris, série Iia. 1995;321:287–94.
Blazy P, Jdid E A. Phénomènes de clinkérisation et de collagelors de la calcination du phosphate à gangue calcaire d’Akashat (Irak). C. R. Acad. Sci. Paris, série IIa. 1997;324:79–86.
Bojinova D. Thermal treatment of Tunisian phosphorite and additives of aluminium silicate. Thermochim Acta. 2003;404:155–62.
Pelovski Y, Petkova V, Dombalov I. Thermal analysis of mechanoactivated mixtures of Tunisia phosphorite and ammonium sulfate. J Therm Anal. 2003;72:967–80.
Slansky M. Geology of sedimentary phosphate. New York: North Oxford Academic; 1986.
Petkova V, Yaneva V. Thermal behavior and phase transformations of nanosized carbonate apatite (Syria). J Therm Anal Calorim. 2010;99:179–89.
Tonsuaadu K, Gross AK, Pluduma L, Veiderma M. A review on the thermal stability of calcium apatites. J Therm Anal Calorim. 2012;110:647–59.
Tonsuaadu K, Peld M, Bender V. Thermal analysis of apatite structure. J Therm Anal Calorim. 2003;72:363–71.
Elliott J. Structure and chemistry of the apatites and other calcium orthophosphates. Amsterdam: Elsevier; 1994.
Fleet ME. Infrared spectra of carbonate apatites: ν2-Region bands. Biomaterials. 2009;30:1473–81.
Liu Y. Review on the vibrational spectroscopy of apatites. J Wuhan Inst of Chem Technol. 2002;1:21–7.
Szilas C, Bender KC, Msolla MM, Borggaard OK. The reactivity of Tanzanian Minjingu phosphate rock can be assessed from the chemical and mineralogical composition. Geoderma. 2008;147:172–7.
Antonakos A, Liarokapis E, Leventouri T. Micro-Raman and FTIR studies of synthetic and natural apatite. Biomaterials. 2007;28:3043–54.
Kaljuvee T, Kuusik R, Veiderma M. Enrichment of carbonate-phosphate ores by calcinations and air separation. Int J Miner Process. 1995;43:113–21.
Knubovets R, Nathan Y, Shoval S, Rabinowitz J. Thermal transformations in phosphorites. J Therm Anal. 1997;50:229–39.
Shoval S, Nathan Y. Analyzing the calcination of sulfur-rich calcareous oil shales using FT-IR spectroscopy and applying curve-fitting technique. J Therm Anal Calorim. 2011;105:883–96.
Daniel BT, Cushla MM, Fordyce RE, Russell DF, Keith CG. Raman spectroscopy of fossil bioapatite—A proxy for diagenetic alteration of the oxygen isotope composition. Palaeogeogra Palaeocl. 2011;310:62–70.
Jehlicka J, Urban O, Pokorny J. Raman spectroscopy of carbon and solid bitumens in sedimentary and metamorphic rocks. Spectrochim Acta A. 2003;59:2341–52.
Awonusi A, Morris MD, Tecklenburg MJM. Carbonate assignment and calibration in the raman spectrum of apatite. J Calcif Tissue Int. 2007;81:46–52.
Penel G, Leroy G, Rey C, Sombret B, Huvenne JP, Bres E. Infrared and Raman microspectrometry study of fluor–fluor-hydroxy and hydroxy-apatite powders. J Mat Sci. 1997;8:271–6.
Zapata F, Roy RN. 2004. Use of phosphate rocks for sustainable agriculture: FAO Fertilizer and plant nutrition bulletin, vol. 13.Rome: FAO;2004.
Panchenko SV, Bobkov VI. Modeling of the heat strengthening of phosphorite pellets. Theor Found Chem Eng. 2002;36:183–7.
Acknowledgements
We thank Nabil Fattah Professor at Research center of Metlaoui in Company of phosphates of Gafsa for providing the phosphorites samples. This work is supported by the Ministry of Higher Education, Scientific Research and Information and Communication Technologies of Tunisia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elgharbi, S., Horchani-Naifer, K. & Férid, M. Investigation of the structural and mineralogical changes of Tunisian phosphorite during calcinations. J Therm Anal Calorim 119, 265–271 (2015). https://doi.org/10.1007/s10973-014-4132-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4132-5