Summary
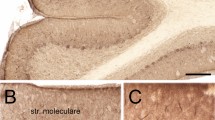
Cerebella of 3- to 6-week-old chickens were cryofixed in a nitrogen-cooled propane jet, deep-etched and rotary-shadowed. The use of a brief perfusion of 0.32 M sucrose improved the quality of the cryofixation and allowed the study of the deeper layers of the cerebellar cortex. It is reported that the cytoskeleton of the Purkinje cells (PC) shows distinct domains and composition of filamentous structures in the different regions of the cell cytoplasm, such as the perikaryon, the cytoplasm of dendrites and the axoplasm. The perikaryon is occupied by a meshwork of fine filaments, 4–7 nm in diameter, that extends from the nuclear outer membrane to the cell membrane. In this zone the cell organelles (e.g., endoplasmic reticulum, mitochondria) adopt a circular arrangement around the nucleus. All structures are anchored by microfilaments to the cytoplasmic network. The dendrites show a dense cytoplasmic network including bundles of microtubules, neurofilaments and microfilaments. Numerous aggregated globular components are attached to this cytoskeleton. The cytoskeleton of the dendritic spines shows axially oriented 10-nm bundles of filaments, which are interconnected and anchored also to the cell membrane and the components of the agranular endoplasmic reticulum by cross-linkers. As described in peripheral nerves, the axoplasm of axons in the central nervous system exhibits predominantly neurofilaments and microtubules aligned along the axis of the neuntes in a three-dimensional arrangement and interconnected by cross-linker filaments and filamentous structures.
Similar content being viewed by others
References
Andres KH (1961) Untersuchungen über den Feinbau von Spinalganglien. Z Zellforsch 55:1–48
Burton PR, Fernandez HL (1973) Delineation by lanthanum staining of filamentous elements associated with the surfaces of axonal microtubules. J Cell Sci 12:567–583
Caceres A, Payne MR, Binder LI, Steward O (1983) Immunocytochemical localization of actin and microtubulc-associated protein MAP2 in dendritic spines. Proc Natl Acad Sci USA 80:1738–1742
Dempsey GP, Bullivant S (1975) A copper block method for freezing non-cryoprotected tissue to produce ice-crystal-free regions for electron microscopy. I. Evaluation using freeze-substitution. J Microsc 106:251–260
Ellisman MH, Porter KR (1980) Microtrabecular structure of the axoplasmic matrix: Visualization of cross-linking structures and their distribution. J Cell Biol 87:464–479
Fine RE, Bray D (1971) Actin in growing nerve cells. Nat New Biol 234:115–118
Fifková E, Delay RJ (1982) Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol 95:345–350
Fifková E, Markham JA, Delay RJ (1983) Calcium in the spine apparatus of dendritic spines in the dentate molecular layer. Brain Res 266:163–168
Geisler N, Weber K (1981) Self-assembly in vitro of the 68,000 molecular weight component of the mammalian neurofilament triplet proteins into intermediate-sized filaments. J Mol Biol 151:565–571
Geisler N, Fischer S, Vandekerckhove J, Van Damme J, Plessmann U, Weber K (1985) Protein-chemical characterization of NF-H, the largest mammalian neurofilament component; intermediate filament-type sequences followed by a unique carboxy-terminal extension. EMBO J 4:57–63
Gershon ND, Porter KR, Trus BL (1985) The cytoplasmic matrix: Its volume and surface area and the diffusion of molecules through it. Proc Natl Acad Sci USA 82:5030–5034
Gray EG (1959) Axo-somatic and axo-dendritic synapses of the cerebral cortex: An electron microscope study. J Anat [Lond] 93:420–433
Gray EG (1973) The cytonet, plain and coated vesicles, reticulosomes, multivesicular bodies and nuclear pores. Brain Res 62:329–335
Gray EG (1975) Synaptic fine structure and nuclear, cytoplasmic and extracellular networks. The stereoframework concept. J Neurocytol 4:315–339
Gray EG (1982) Rehabilitating the dendritic spine. TINS 5:5–6
Heuser JE, Kirschner MW (1980) Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol 86:212–234
Hillman DE (1969) Neuronal organization of the cerebellar cortex in Amphibia and Reptilia. In: Llinás R (ed) Neurobiology of Cerebellar Evolution and Development. American Medical Association, Chicago, Ill, pp 279–325
Hillman DE (1979) Neuronal shape parameters and substructures as a basis of neuronal form. In: Schmitt FO, Worden FG (eds) The Neurosciences. Fourth Study Program. The MIT Press, Cambridge London, pp 477–498
Hirokawa N (1982) Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol 94:129–142
Hirokawa N, Glicksman MA, Willard MB (1984) Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol 98:1523–1536
Hirokawa N, Bloom GS, Vallee RB (1985) Cytoskeletal architecture and immunocytochemical localization of microtubule-associated proteins in regions of axons associated with rapid axonal transport: The β,β′-iminodipropionitrile-intoxicated axon as a model system. J Cell Biol 101:227–239
Hoffman PN, Lasek RJ (1975) The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol 66:351–366
Landis DMD, Reese TS (1974) Differences in membrane structure between excitatory and inhibitory synapses in the cerebellar cortex. J Comp Neurol 155:93–126
Landis DMD, Reese TS (1977) Structure of the Purkinje cell membrane in staggerer and weaver mutant mice. J Comp Neurol 171:247–260
Landis DM, Reese TS (1983) Cytoplasmic organization in cerebellar dendritic spines. J Cell Biol 97:1169–1178
Lasek RJ (1980) Dynamic properties of cytoskeletons. The dynamic ordering of neuronal cytoskeletons. Neurosci Res Prog Bull 19:7–32
Lasek RJ, Oblinger MM, Drake PF (1983) Molecular biology of neuronal geometry: Expression of neurofilament genes influences axonal diameter. Cold Spring Harbor Symp Quant Biol 48:731–744
Lasek RJ, Garner JA, Brady ST (1984) Axonal transport of the cytoplasmic matrix. J Cell Biol 99:212s-221s
LeBeaux YJ, Willemot J (1975a) An ultrastructural study of the microfilaments in rat brain by means of HMM labeling. I. The perikaryon, the dendrites and the axon. Cell Tissue Res 160:1–36
Le Beaux YJ, Willemot J (1975b) An ultrastructural study of the microfilaments in rat brains by means of E-PTA staining and HMM labeling. II. The synapses. Cell Tissue Res 160:37–68
Levine J, Willard M (1981) Fodrin: Axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol 90:631–643
Liem RKH, Yen S-H, Salomon GD, Shelanski ML (1978) Intermediate filaments in nervous tissues. J Cell Biol 79:637–645
Margaritis LH, Elgsaeter A, Branton D (1977) Rotary replication for freeze-etching. J Cell Biol 72:47–56
Matus A, Bernhardt R, Hugh-Jones T (1981) High molecular weight microtubule associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci USA 78:3010–3014
Matus A, Ackermann M, Pehling G, Byers RH, Fujiwara K (1982) High actin concentration in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci USA 79:7590–7594
Meller K (1984) The ultrastructure of the developing inner and outer segments of the photoreceptors of chick embryo retina as revealed by the rapid-freezing and deep-etching technique. Anat Embryol 169:141–150
Meller K (1985a) Ultrastructural aspects of the choroid plexus epithelium as revealed by the rapid-freezing and deep-etching techniques. Cell Tissue Res 239:189–201
Meller K (1985b) Ultrastructural aspects of cryofixed nerves. Cell Tissue Res 242:289–300
Metuzals J, Mushynski WE (1974) Electron microscope and experimental investigations of the neurofilamentous network in Deiters' neurons. Relationship with the cell surface and nuclear pores. J Cell Biol 61:701–722
Metuzals J, Tasaki I (1978) Subaxolemmal filamentous network in the giant nerve fiber of the squid (Loligo pealei 1.) and its possible role in excitability. J Cell Biol 78:597–621
Metuzals J, Montpetit V, Clapin DF (1981) Organization of the neurofilamentous network. Cell Tissue Res 214:455–482
Metuzals J, Hodge AJ, Lasek RJ, Kaiserman-Abramof IR (1983) Neurofilamentous network and filamentous matrix preserved and isolated by different techniques from squid giant axon. Cell Tissue Res 228:415–432
Moon HM, Wisniewski T, Merz P, De Martini J, Wisniewski HM (1981) Partial purification of neurofilament subunits from bovine brains and studies on neurofilament assembly. J Cell Biol 89:560–567
Moor H, Kistler J, Müller M (1976) Freezing in a propane jet. Experientia 32:805
Ornberg RL, Reese TS (1979) Artifacts of freezing in Limulus amebocytes. In: Rash JE, Hudson CS (eds) Freeze Fracture: Methods, Artifacts, and Interpretations. Raven Press, New York, pp 89–97
Palay SL, Chan-Palay V (1974) Cerebellar Cortex. Cytology and Organization. Springer, Berlin Heidelberg New York
Palay SL, Chan-Palay V (eds) (1982) The Cerebellum — New Vistas. Springer, Berlin Heidelberg New York
Pappas GD, Purpura DP (1961) Fine structure of dendrites in the superficial neocortical neuropil. Exp Neurol 4:507–530
Porter KR (1984) The cytomatrix: A short history of its study. J Cell Biol 99:3s-12s
Porter KR, Anderson KL (1982) The structure of the cytoplasmic matrix preserved by freeze-drying and freeze-substitution. Eur J Cell Biol 29:83–96
Porter KR, McNiven MA (1982) The cytoplast: A unit structure in chromatophores. Cell 29:23–32
Porter KR, Tucker JB (1981) The ground substance of the living cell. Sci Am 244:40–51
Porter KR, Byers HR, Ellisman MH (1979) The cytoskeleton. In: Schmitt FO, Worden FG (eds) The Neurosciences. Fourth Study Program. The MIT Press, Cambridge, Mass London, England, pp 703–722
Pumplin DW, Reese TS, Llinás R (1981) Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci USA 78:7210–7213
Ramón y Cajal S (1891) Sur la structure de l'écorce cérébrale de quelques mammifères. Cellule 7:125–176
Schlaepfer WW (1977) Studies on the isolation and substructure of mammalian neurofilaments. J Ultrastruct Res 61:149–157
Schlaepfer WW, Freeman LA (1978) Neurofilament proteins of rat peripheral nerve and spinal cord. J Cell Biol 78:653–662
Schlaepfer WW, Lee V, Wu H-L (1981) Assessment of immunological properties of neurofilament triplet proteins. Brain Res 226:259–272
Schnapp BJ, Reese TS (1982) Cytoplasmic structure in rapid-frozen axons. J Cell Biol 94:667–679
Sleytr UB, Robards AW (1977) Plastic deformation during freezecleavage: a review. J Microsc 110:1–25
Sotelo C (1969) Ultrastructural aspects of the cerebellar cortex of the frog. In: Llinás R (ed) Neurobiology of Cerebellar Evolution and Development. American Medical Association, Chicago, Ill, pp 327–371
Tokuyasu KT (1984) Immuno-cryoultramicrotomy. In: Polak JM, Varndell IM (eds) Immunolabelling for Electron Microscopy. Elsevier, Amsterdam New York Oxford, pp 71–82
Tsukita S, Ishikawa H (1981) The cytoskeleton in myelinated axons: Serial section study. Biomed Res 2:424–437
Tsukita S, Usukura J, Tsukita S, Ishikawa H (1982) The cytoskeleton in myelinated axons: A freeze-etch replica study. Neuroscience 7:2135–2147
Van Harreveld A, Crowell J, Malhotra SK (1965) A study of extracellular space in central nervous tissue by freeze-substitution. J Cell Biol 25:117–137
Westrum LE, Hugh Jones D, Gray EG, Barron J (1980) Microtubules, dendritic spines and spine apparatuses. Cell Tissue Res 208:171–181
Willard M, Simon C (1981) Antibody decoration of neurofilaments. J Cell Biol 89:198–205
Willard M, Simon C (1983) Modulations of neurofilament axonal transport during the development of rabbit retinal ganglion cells. Cell 35:551–559
Willard M, Simon C, Baitinger C, Levine J, Skene P (1980) Association of an axonally transported polypeptide (H) with 100-Å filaments. Use of immunoaffinity electron microscope grids. J Cell Biol 85:587–596
Wolosewick JJ, Porter KR (1976) Stereo high-voltage electron microscopy of whole cells of the human diploid cell line, WI-38. Am J Anat 147:303–324
Wolosewick JJ, Porter KR (1979) Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol 82:114–139
Yamada KM, Spooner BS, Wessells NK (1971) Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol 49:614–635
Zackroff RV, Idler WW, Steinert PM, Goldman RD (1982) In vitro reconstitution of intermediate filaments from mammalian neurofilament triplet polypeptides. Proc Natl Acad Sci USA 79:754–757
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meller, K. The cytoskeleton of cryofixed Purkinje cells of the chicken cerebellum. Cell Tissue Res. 247, 155–165 (1987). https://doi.org/10.1007/BF00216558
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216558