Summary
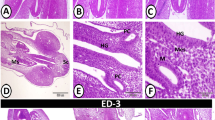
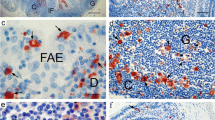
Dome epithelium (DE), the tissue covering lymphoid domes of gut-associated lymphoid tissues, was examined in both adult and neonatal rabbit appendix or sacculus rotundus to determine if dome epithelial cells matured earlier than epithelial cells covering adjacent villi. The localization of well-differentiated epithelial cells in rabbit gut-associated lymphoid tissues (GALT) was accomplished histochemically by use of molecular probes: fluorescein isothiocyanate or horseradish peroxidase conjugates of Ulex europaeus agglutinin I (UEA), a lectin specific for terminal L-fucose molecules on certain glycoconjugates. The villus epithelial cells of newborn and 2-, 5-, or 10-day-old rabbits did not bind UEA, but between the twelfth and fifteenth days of postnatal life, UEA receptors were expressed by well-differentiated villus epithelial cells. In contrast to villus epithelium, DE in appendix and sacculus rotundus of neonatal rabbits expressed UEA receptors two days after birth, a feature that distinguished the DE of neonatal GALT for the next two weeks. In adult rabbits, UEA receptors were associated with dome epithelial cells extending from the mouths of glandular crypts to the upper domes; in contrast to the domes, UEA receptors were only present on well-differentiated epithelial cells at the villus tips. Results suggested that in neonatal rabbits most dome epithelial cells developed UEA receptors shortly after birth, reflecting precocious development of DE as compared to villus epithelium. In adult rabbit dome epithelium UEA receptors appeared on dome epithelial cells as they left the glandular crypts, representing accelerated epithelial maturation.
Similar content being viewed by others
Abbreviations
- DE:
-
dome epithelium
- DEL:
-
dome epithelial lymphocytes
- FITC:
-
fluorescein isothiocyanate
- HRP:
-
horseradish peroxidase
- PBS:
-
phosphate-buffered saline
- PBS-CaMg:
-
PBS containing calcium and magnesium ions
- UEA:
-
Ulex europaeus agglutinin I
References
Avrameas S (1969) Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry 6:43–52
Berendson R, Boedeker EC, Cheney CP (1982) Age related changes in lectin binding to the rabbit intestinal mucosa: evidence for the evolution of complex carbohydrate surface structures with weaning. Gastroenterology 82:1016 (Abstract)
Bhalla DK, Owen RL (1982) Cell renewal and migration in lymphoid follicles of Peyer's patches and cecum —an autoradiographic study in mice. Gastroenterology 82:232–242
Bhalla DK, Owen RL (1983) Migration of T and B lymphocytes to M cells in Peyer's patch follicle epithelium: an autoradiographic and immunocytochemical study in mice. Cell Immunol 81:105–117
Bienenstock J, Befus D (1984) Gut- and bronchus-associated lymphoid tissues. Am J Anat 170:437–445
Bockman DE, Cooper MD (1973) Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix and Peyer's patches. An electron microscopic study. Am J Anat 136:455–478
Boland CR, Ahnen DJ (1985) Binding of lectins to goblet cell mucin in malignant and premalignant colonic epithelium in the CF-1 mouse. Gastroenterology 89:127–137
Bye WA, Allen CH, Trier JS (1984) Structure, distribution, and origin of M cells in Peyer's patches of mouse iluem. Gastroenterology 86:789–801
Cerf-Bensussan N, Quaroni A, Kurnick JT, Bhan AK (1974) Intraepithelial lymphocytes modulate Ia expresssion by intestinal epithelial cells. J Immunol 132:2244–2252
Etzler ME, Branstrator ML (1974) Differential localization of cell surface and secretory components in rat intestinal epithelium by use of lectins. J Cell Biol 62:329–337
Farr AG, Anderson SK (1985) Epithelial heterogeneity in the murine thymus: fucose-specific lectins bind medullary epithelial cells. J Immunol 134:2971–2977
Fischer J, Klein PJ, Vierbuchen M, Skutta B, Uhlenbruck G, Fischer R (1984) Characterization of glycoconjugates of human gastrointestinal mucosa by lectins. 1. Histochemical distribution of lectin binding sites in normal alimentary tract as well as in benign and malignant gastric neoplasms. J Histochem Cytochem 32:681–689
Goldstine SN, Manickavel V, Cohen N (1975) Phylogeny of gutassociated lymphoid tissue. Am Zool 15:107–118
Holthofer H, Virtanen I, Kariniemi AL, et al. (1982) Ulex europeaus I lectin as a marker for vascular endothelium in human tissues. Lab Invest 47:60–66
Kuhlmann WD, Peschke P, Wurster K (1983) Lectin-peroxidase conjugates in histopathology of gastrointestinal mucosa. Virchows Arch [Pathol Anat] 398:319–327
Madara JL, Bye WA, Trier JS (1984) Structural features of and cholesterol distribution in M-cell membranes in guinea pig, rat, and mouse Peyer's patches. Gastroenterology 87:1091–1103
Mayrhofer G, Pugh CW, Barclay AN (1983) The distribution, on togeny and origin in the rat of Ia-positive cells with dendritic morphology and Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol 13:112–122
Namanic MK, Whitehead JS, Elias PM (1983) Alterations in membrane sugars during epidermal differentiation: visualization with lectins and role of glycosidases. J Histochem Cytochem 31:887–897
Neutra MR, Guerina NG, Hall TL, Nicolson GL (1982) Transport of membrane bound molecules by M cells in rabbit intestine. Gastroenterology 82:1137 (abstract)
Niewenhuis P (1971) The Origin and Fate of Immunologically Competent Cells. Wolters-Noordhoff, Groningen, NL
Obek I, Mirelman D, Sharon N (1977) Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature 265:623–625
Owen RL (1977) Sequential uptake of horseradish peroxidase by lymphoid follicle of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology 72:440–451
Owen RL, Jones AL (1974) Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology 66:189–203
Owen RL, Bhalla DK (1983) Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer's patch cells. Am J Anat 186:199–212
Owen RL, Pierce NF, Apple RT, Cray WC Jr (1986) M cell transport of Vibrio choleras from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis 153:1108–1118
Pereira MEA, Kisailus EC, Gruezo F, Kabat EA (1978) Immunohistochemical studies on the combining site of the blood group H-specific lectin 1 from Ulex europeus seeds. Arch Biochem Biophys 185:108–115
Prime SS, Rosser TJ, Malamos D, Shepherd JP, Scully C (1985) The use of the lectin Ulex europaeus to study epithelial cell differentiation in neoplastic and non-neoplastic oral white lesions. J Path 147:173–179
Quaroni A (1985) Crypt cell development in newborn rat small intestine. J Cell Biol 100:1601–1610
von Rosen L, Podjaski B, Bettmann I, Otto HF (1981) Observations on the ultrastructure and function of the so-called “microfold” or “membranous” cells (M cells) by means of peroxidase as a tracer. Virchows Arch [Path Anat] 390:289–312
Roy MJ, Ruiz A (1986) Dome epithelial M cells dissociated from rabbit gut-associated lymphoid tissues. Am J Vet Res 47:2577–2583
Roy MJ, Ruiz A, Varvayanis M (1987) A novel antigen is common to the dome epithelium of gut- and bronchus-associated lymphoid tissues. Cell Tiss Res 248:635–644
Schmidt GH, Wilkinson MM, Ponder BAJ (1985) Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell 40:425–429
Sell S, Linthicum DS, Bass D, Bahu R, Wilson B, Nakane P (1977) Immunohistologic technics. In: Borek C et al. (eds) Cancer Biology. Vol IV. Differentiation and Cancinogenesis. Stratton, NY, pp 272–305
Sharon N (1984) Lectin-like bacterial adherence to animal cells. In: Boedeker EC (ed) Attachment of Organisms to Gut Muosa, Vol 1. CRC Press, Boca Raton, FL, pp 129–147
Smith MW, Peacock MA (1980) “M” cell distribution in follicle-associated epithelium of mouse Peyer's patch. Am J Anat 159:167–175
Smith MW, Jarvis LG, King IS (1980) Cell proliferation in follicle-associated epithelium of mouse Peyer's patch. Am J Anat 159:157–166
Torres-Medina A (1981) Morphologic characteristic of the epithelial surface of aggregated lymphoid follicles (Peyer's patches) in the small intestine of newborn gnotobiotic calves and pigs. Amer J Vet Res 42:232–236
Virtanen I, Lehtonen E, Narvanan O, Leivo I, Lehto V-P (1985) Population heterogeneity in the surface expression of Ulex europaeus I-lectin (UEA1)-binding sites in cultured malignant and transformed cells. Exp Cell Res 161:53–62
Wolf JL, Kauffman RS, Finberg R, Dambrauskas R, Field BN, Trier JS (1983) Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology 85:291–300
Zieske JD, Bernstein IA (1982) Modification of cell surface glycoprotein: addition of fucosyl residues during epidermal differentiation. J Cell Biol 95:626–631
Author information
Authors and Affiliations
Additional information
The views of the authors expressed here do not purport to reflect the position of the Department of the Army or the Department of Defense
In conducting the research described in this report, the investigators adhered to standards set forth in the “Guide for the Care and Use of Laboratory Animals” (NIH Publication 85-23) as promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, USA
Rights and permissions
About this article
Cite this article
Roy, M.J. Precocious development of lectin (Ulex europaeus agglutinin I) receptors in dome epithelium of gut-associated lymphoid tissues. Cell Tissue Res. 248, 483–489 (1987). https://doi.org/10.1007/BF00216473
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216473