Abstract
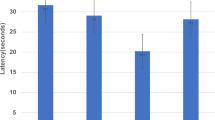
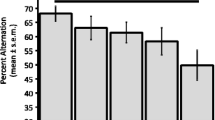
Numerous studies suggest that age-related declines in memory storage are related to impairment of central cholinergic systems. Scopolamine, a muscarinic cholinergic antagonist, has been used with young humans and other animal species as a model of the cognitive impairment that often accompanies normal and pathological aging. The present study examined whether amnesia induced by scopolamine could be counteracted in mice by arecoline, a cholinergic agonist, or by other drugs, epinephrine or glucose, which have been found to enhance memory in aged rodents and humans. Young mice were administered scopolamine (3 mg/kg, IP) or saline prior to training on an inhibitory avoidance apparatus. Immediately after training, animals received injections of epinephrine (0.01, 0.05, 0.1, and 0.2 mg/kg), glucose (10, 100, and 250 mg/kg), arecoline (0.5, 1, 2, 5, 10, and 20 mg/kg), or saline. The results indicate that pre-training scopolamine reliably impaired retention assessed in test trials 48 h after training. This impairment was not attentuated by any post-training dose of arecoline; however, immediate post-training injections of both epinephrine (at 0.05 mg/kg) and glucose (at 100 mg/kg) significantly reduced the amnesia. Neither of these drugs was effective if injections were delayed by 1 h after training. These results support the value of scopolamine as a model of age-related memory impairments, but suggest further that these memory deficits may be particularly susceptible to attenuation with non-cholinergic treatments.
Similar content being viewed by others
References
Bartus RT, Dean RL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction: a critical review. Science 217:408–417
Christie JE, Shering A, Ferguson J, Glen AIM (1981) Physostigmine and arecoline: effects of intravenous infusions in Alzheimer's presenile dementia. Br J Psychiatry 138:46–50
Dastur DK, Lane MH, Hansen DB, Kety SS, Butler RN, Perlin S, Sokoloff L (1963) Effects of aging on cerebral circulation and metabolism. In: Birren JE, Butler RN, Greenhouse SW, Sokoloff L, Yarrow MR (eds) Human aging, a biological and behavioral study. U.S.P.H.S. Publ. 986, US Government Printing Office, Washington DC, pp 57–76
Davies P, Maloney AJF (1976) Selective loss of central cholinergic neurons in Alzheimer's disease (letter). Lancet II:1043
Davis KL, Yamamura HI (1978) Cholinergic underactivity in human memory disorders. Life Sci 23:1729–1734
Drachman DA, Leavitt J (1974) Human memory and the cholinergic system: a relationship to aging? Arch Neurol 30:113–121
Ferris SH, Sathananthan G, Reisberg B, Gershon S (1979) Longterm choline treatment of memory-impaired elderly patients. Science 205:1039–1040
Flood JF, Cherkin A (1986) Scopolamine effects on memory retention in mice: a model of dementia? Behav Neural Biol 45:169–184
Gold PE (1984) Memory modulation: roles of peripheral catecholamines. In: Squire L, Butters N (eds) The neuropsychology of memory. Guilford Press, New York, pp 566–578
Gold PE (1986) Glucose modulation of memory storage. Behav Neural Biol 45:342–349
Gold PE, van Buskirk R (1978a) Posttraining brain norepinephrine concentrations: correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behav Biol 23:509–520
Gold PE, van Buskirk R (1978b) Effects of α- and β-adrenergic receptor antagonists on post-trial epinephrine modulation of memory: relationship to post-training brain norepinephrine concentrations. Behav Biol 24:168–184
Gold PE, Zornetzer SF (1983) The mnemon and its juices: neuromodulation of memory processes. Behav Neural Biol 38:151–189
Gold PE, Vogt J, Hall JL (1986) Glucose effects on memory: behavioral and pharmacological characteristics. Behav Neural Biol 46:145–155
Hall JL, Gold PE (1986) The effects of training, epinephrine, and glucose injections on plasma glucose levels in rats. Behav Neural Biol 46:156–167
Hall JL, Gonder-Frederick L, Vogt J, Gold PE (1986) Glucose enhancement of memory in aged humans. Soc Neurosci Abst 12:1312
Krnjevic K (1974) Chemical nature of synaptic transmission in vertebrates. Physiol Rev 54:418–540
Kubanis P, Zornetzer SF (1981) Age-related behavioral and neurobiological changes: a review with emphasis on memory. Behav Neural Biol 31:115–172
McGaugh JL, Gold PE (1988) Hormonal modulation of memory. In: Levine S, Brush R (eds) Psychoendocrinology, Academic Press, New York (in press)
Mohns RC, Davis KL, Tinklenberg JR, Hollister L (1980) Choline chloride effects on memory in the elderly. Neurobiol Aging 1:21–25
Morley JE (1986) Neuropeptides, behavior, and aging. J Am Geriatr Soc 34:52–62
Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH (1978) Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J II:1457–1459
Rossor M, Iversen LL (1986) Non-cholinergic neurotransmitter abnormalities in Alzheimer's disease. Br Med Bull 42:70–74
Rush DK (1986) Reversal of scopolamine induced amnesia of passive avoidance by pre- and post-training naloxone. Psychopharmacology 89:296–300
Smith CB, Goochee C, Rapoport SI, Sokoloff L (1980) Effects of ageing on local rates of cerebral glucose utilization in the rat. Brain 103:351–365
Sternberg DB, Martinez J, McGaugh JL, Gold PE (1985) Agerelated memory deficits in rats and mice: enhancement with peripheral injections of epinephrine. Behav Neural Biol 44:213–220
Stone WS, Cottrill KL, Gold PE (1987) Glucose and epinephrine attenuation of scopolamine-induced increases in locomotor activity in mice. Neurosci Res Commun 1:105–111
Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR (1981) Alzheimer's disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 10:122–126
Zornetzer SF (1984) Brain substrates of senescent memory decline. In: Squire L, Butters N (eds) The neuropsychology of memory. Guilford Press, New York, pp 588–600
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stone, W.S., Croul, C.E. & Gold, P.E. Attenuation of scopolamine-induced amnesia in mice. Psychopharmacology 96, 417–420 (1988). https://doi.org/10.1007/BF00216073
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00216073