Abstract
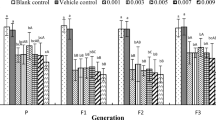
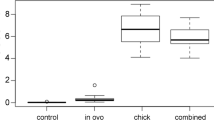
A two-generation laboratory study was conducted on a captive population of American kestrels (Falco sparverius) to investigate the possible reproductive and teratogenic effects of the pesticide dicofol. Paired females were exposed to three levels of dicofol: 0, 5, and 20 mg/kg. Integrity of the reproductive tract of the resulting embryos was examined. Viable eggs were hatched, and these birds were permitted to breed the following year. Breeding performance for these birds was measured based on their ability to form pair bonds and exhibit normal behavior in the presence of a mate. Clutch completion, fertility, hatchability, and number of hatchlings reared to the fledging were used as reproductive parameters. Females dosed with 20 mg/kg of dicofol laid eggs with shells that were significantly (p<0.05) thinner than those of the control birds. Residue levels of dicofol in the form of dichlorobenzophenone were detected in the first and second clutch eggs of the 20-mg/kg dose group only. Male embryos from females dosed with 5 and 20 mg/kg of dicofol had gonads that were significantly different (p<0.05) from the control chicks. Feminization of male embryos was confirmed by the presence of primordial germ cells in the male gonad. Second-generation adult 5-mg/kg females showed a significantly (p<0.05) greater number of eggs and hatched chicks lost when compared to second-generation control females. Similar results were found in second-generation 5-mg/kg males paired with normal females and had a significant (p<0.05) number of chicks die posthatching. Results of second-generation breeding parameters indicate a negative effect on reproductive behavior.
Similar content being viewed by others
References
Adkins EK (1975) Hormonal basis of sexual differentiation in the Japanese quail. J Comp Physiol Psychol 89:61–71
Bennett JK, Dominguez SE, Griffis WL (1990) Effects of dicofol on Mallard eggshell quality. Arch Environ Contam Toxicol 19:907–912
Berger DD, Anderson DW, Weaver JD, Risebrough RW (1970) Shell thinning in eggs of Ungava peregrines. Can Field Nat 84:265–267
Bird DM (1985) Evaluation of the American kestrel (Falco sparverius) as a laboratory research animal. In: Archibald J, Ditchfield J, Rowsell HC (eds) The contribution of laboratory animal science to the welfare of man and animals. Gustav Fischer Verlag, Stuttgart, New York, pp 3–9
Bryan TE, Gildersleeve RP, Wiard RP (1989) Exposure of Japanese Quail embryos to o,p′-DDT has long-term effects on reproductive behaviours, hematology, and feather morphology. Teratology 39:525–535
Clark DR, Spann JW, Bunck CM (1990) Dicofol (KelthaneR)-induced eggshell thinning in captive American kestrels. Environ Toxicol Chem 9:1063–1069
Conover MR (1984) Occurrence of supernormal clutches in the laridae. Wilson Bull 96:249–267
Fry M, Toone CK (1981) DDT-Induced feminization of gull embryos. Science 213:922–924
Fry M, Toone CK, Speich SM, Peard RJ (1987) Sex ratio skew and breeding patterns of gulls: Demographic and toxicological considerations. Stud Avian Biol 10:26–43
Fry M, Rossen B, Bombardier M, Ditto M, MacLellan K, Bird DM (1988) Eggshell thinning and embryo toxicity of dicofol miticide to American kestrels. Platform presentation, SETAC 9th Annual Meeting, Nov 1988, Washington, DC
Fry M, Schwarzbach SE, Lasley BL (1989) Detection of active metabolites of chlorinated hydrocarbons and effects on avian reproduction. Unpublished data, Ecology project #2, University of California at Davis
Hickey JJ, Anderson DW (1968) Chlorinated hydrocarbons and eggshell changes in raptorial and fish-eating birds. Science 162:271–273
Hunt GL, Wingfield JC, Newman A, Farner DS (1980) Sex ratios of western gulls (Larus occidentalis) in southern California. Auk 97:473–479
Lutz-Ostertag Y, David D (1973) Action du DDT sur le tractus urogénital de l'embryon de Poulet et de Caille. C R Acad Sc (Paris), Série D276:1213–1216
Mineau P, Fox GA, Norstrom RJ, Weseloh DV, Hallett DJ, Ellenton JA (1984) Using the Herring Gull to monitor levels and effects of organochlorine in the Canadian Great Lakes. In: Nriagu JO, Simmons MS (eds) Toxic contaminants in the Great Lakes. J Wiley and Sons, New York, pp 425–452
Mourer CR, Hall G, Whitehead W, Shibamoto T, Shull L, Schwarzbach S (1990) Chromatographic determination of Dicofol and metabolites in egg yolks. Arch Environ Contam Toxicol 19:154–156
Peakall DB (1970) p,p′-DDT: Effect on calcium metabolism and concentration of estradiol in the blood. Science 168:592–594
Peakall DB (1975) Physiological effects of chlorinated hydrocarbons on avian species. In: Haque R, Freed VH (eds). Environmental dynamics of pesticides. Plenum, New York, pp 343–360
Porter RD, Wiemeyer SN (1970) Propagation of captive American kestrels. J Wildl Manage 34:594–604
Ratcliffe DA (1967) Decreased eggshell weight in certain birds of prey. Nature 215:208–210
Rattner BA, Eroschenko VP, Fox GA, Fry DM, Gorsline J (1984) Avian endocrine responses to environmental pollutants. J Exper Zool 232:683–689
Romanoff AL (1960) The avian embryo. Macmillan Co, New York
Schwarzbach SE, Shull L, Grau CR (1988) Eggshell thinning in Ring Doves exposed to p,p′-dicofol. Arch Environ Contam Toxicol 17:219–227
Speich SM, Calambokidas J, Shea DW, Peard J, Witter M, Fry DM (1992) Eggshell thinning and organochlorine contaminants in West Washington waterbirds. Colonial Waterbirds 15:103–112
USEPA (1984) Dicofol: Special review document 2/3. USEPA, Office of Pesticides and Toxic Substances, Washington, DC, 148 pp
Wiemeyer S, Porter RD (1970) DDE thins eggshells of captive American Kestrels. Nature 227:737–738
Wiemeyer S, Spann JW, Bunck CM, Krynitsky J (1989) Effects of kelthane on reproduction of screech owls. Environ Toxicol Chem 8(10):903–913
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MacLellan, K.N.M., Bird, D.M., Fry, D.M. et al. Reproductive and morphological effects of o,p′-dicofol on two generations of captive American kestrels. Arch. Environ. Contam. Toxicol. 30, 364–372 (1996). https://doi.org/10.1007/BF00212295
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00212295