Summary
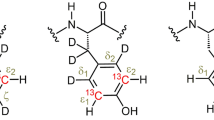
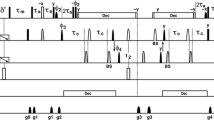
Dynamics of the backbone and some side chains of apo-neocarzinostatin, a 10.7 kDa carrier protein, have been studied from 13C relaxation rates R1, R2 and steady-state 13C-{1H} NOEs, measured at natural abundance. Relaxation data were obtained for 79 nonoverlapping Cα resonances and for 11 threonine Cβ single resonances. Except for three Cα relaxation rates, all data were analysed from a simple two-parameter spectral density function using the model-free approach of Lipari and Szabo. The corresponding C−H fragments exhibit fast (τe < 40 ps) restricted libration motions (S2=0.73 to 0.95). Global examination of the microdynamical parameters S2 and τe along the amino acid sequence gives no immediate correlation with structural elements. However, different trends for the three loops involved in the binding site are revealed. The β-ribbon comprising residues 37 to 47 is spatially restricted, with relatively large τe values in its hairpin region. The other β-ribbon (residues 72 to 87) and the large disordered loop ranging between residues 97–107 experience small-amplitude motions on a much faster (picosecond) time scale. The two N-terminal residues, Ala1 and Ala2, and the C-terminal residue Asn113, exhibit an additional slow motion on a subnanosecond time scale (400–500 ps). Similarly, the relaxation data for eight threonine side-chain Cβ must be interpreted in terms of a three-parameter spectral density function. They exhibit slower motions, on the nanosecond time scale (500–3000 ps). Three threonine (Thr65, Thr68, Thr81) side chains do not display a slow component, but an exchange contribution to the observed transverse relaxation rate R2 could not be excluded at these sites. The microdynamical parameters (S2, τe and R2ex) or (S sup2infslow , S sup2inffast and τslow) were obtained from a straightforward solution of the equations describing the relaxation data. They were calculated assuming an overall isotropic rotational correlation time τe for the protein of 5.7 ns, determined using standard procedures from R2/R1 ratios. However, it is shown that the product (1−S2)× τe is nearly independent of τe for residues not exhibiting slow motions on the nanosecond time scale. In addition, this parameter very closely follows the heteronuclear NOEs, which therefore could be good indices for local fast motions on the picosecond time scale.
Similar content being viewed by others
References
Abragam, A. (1961) Les Principes du Magnétisme Nucléaire, Presses Universitaires de France, Paris.
Adjadj, É., Mispelter, J., Quiniou, É., Dimicoli, J.L., Favaudon, V. and Lhoste, J.M. (1990) Eur. J. Biochem., 190, 263–271.
Adjadj, É., Quiniou, É., Mispelter, J., Favaudon, V. and Lhoste, J.M. (1992a) Eur. J. Biochem., 203, 505–511.
Adjadj, É., Quiniou, É., Mispelter, J., Favaudon, V. and Lhoste, J.M. (1992b) Biochimie, 74, 853–858.
Arvidsson, K., Jarvet, J., Allard, P. and Ehrenberg, A. (1994) J. Biomol. NMR, 4, 653–672.
Barbato, G., Ikura, M., Kay, L.E., Pastor, R.W. and Bax, A. (1992) Biochemistry, 31, 5269–5278.
Berglund, H., Kovacs, H., Dahlman-Wright, K., Gustafsson, J.A. and Härd, T. (1992) Biochemistry, 31, 12001–12011.
Blackledge, M.L., Brüshweiler, R., Griesinger, C., Schmidt, J.M., Xu, P. and Ernst, R.R. (1993) Biochemistry, 32, 10960–10974.
Cantor, C.R. and Schimmel, P.R. (1980) Biophysical Chemistry, Vol. II, Freeman, New York, NY, pp. 459–463.
Careri, G., Fasella, P. and Gratton, E. (1975) CRC Crit. Rev. Biochem., 3, 141–164.
Cheng, J.W., Lepre, C.A., Chambers, S.P., Fulghum, J.R., Thomson, J.A. and Moore, J.M. (1993) Biochemistry, 32, 9000–9010.
Clore, G.M., Szabo, A., Bax, A., Kay, L.E., Driscoll, P.C. and Gronenborn, A.M. (1990a) J. Am. Chem. Soc., 112, 4989–4991.
Clore, G.M., Driscoll, P.C., Wingfield, P.T. and Gronenborn, A.M. (1990b) Biochemistry, 29, 7387–7401.
Constantine, K.L., Friedrichs, M.S., Goldfarb, V., Jeffrey, P.D., Sheriff, S. and Mueller, L. (1993) Proteins, 15, 290–311.
Daragan, V.A., Kloczewiak, M.A. and Mayo, K.H. (1993) Biochemistry, 32, 10580–10590.
Dellwo, M.J. and Wand, A.J. (1989) J. Am. Chem. Soc., 111, 4571–4578.
Deverell, C., Morgan, R.E. and Strange, J.H. (1970) Mol. Phys., 18, 553–559.
Farrow, N.A., Muhandiram, R., Singer, A.U., Pascal, S.M., Kay, C.M., Gish, G., Shoelson, S.E., Pawson, T., Forman-Kay, J.D. and Kay, L.E. (1994) Biochemistry, 33, 5984–6003.
Favaudon, V. (1983) Biochimie, 65, 593–607.
Frauenfelder, H., Sligar, S.G. and Wolynes, P.G. (1991) Science, 254, 1598–1603.
Fushman, D., Weisemann, R., Thüring, H. and Rüterjans, H. (1994) J. Biomol. NMR, 4, 61–78.
Grasberger, B.L., Gronenborn, A.M. and Clore, G.M. (1993) J. Mol. Biol., 230, 364–372.
Jones, D.N.M., Searles, M.A., Shaw, G.L., Churchill, M.E.A., Ner, S.S., Keeler, J., Travers, A.A. and Neuhaus, D. (1994) Structure, 2, 609–627.
Kappen, L.S., Napier, M.A. and Goldberg, I.H. (1980) Proc. Natl. Acad. Sci. USA, 77, 1970–1974.
Karplus, M. (1986) Methods Enzymol., 131, 283–307.
Kay, L.E., Jue, T.L., Bangerter, B. and Demou, P.C. (1987) J. Magn. Reson., 73, 558–564.
Kay, L.E., Torchia, D.A. and Bax, A. (1989) Biochemistry, 28, 8972–8979.
Kay, L.E., Nicholson, L.K., Delaglio, F., Bax, A. and Torchia, D.A. (1992) J. Magn. Reson., 97, 359–375.
Kelsh, P.L., Ellena, J.F. and Cafiso, D.S. (1992) Biochemistry, 31, 5136–5144.
Kemple, M.D., Yuan, P., Nollet, K.E., Fuchs, J.A., Silav, N. and Prendergast, F.G. (1994) Biophys. J., 66, 2111–2126.
Kim, K.-H., Kwon, B.-M., Myers, A.G. and Rees, D.C. (1993) Science, 262, 1042–1045.
Kraulis, P.J. (1991) J. Appl. Crystallogr., 24, 946–950.
Lefèvre, C., Adjadj, É., Quiniou, É. and Mispelter, J. (1994) J. Biomol. NMR, 4, 689–702.
Lepre, C.A., Cheng, J.-W. and Morre, J.M. (1993) J. Am. Chem. Soc., 115, 4929–4930.
Lipari, G. and Szabo, A. (1982) J. Am. Chem. Soc., 104, 4546–4570.
London, R.E. (1989) Methods Enzymol., 176, 358–375.
McCammon, J.A. and Harvey, S.C. (1987) Dynamics of Proteins and Nucleic Acids, Cambridge University Press, Cambridge.
Nicholson, L.K., Kay, L.E., Baldisseri, D.M., Arango, J., Young, P.E., Bax, A. and Torchia, D.A. (1992) Biochemistry, 31, 5253–5263.
Nirmala, N.R. and Wagner, G. (1988) J. Am. Chem. Soc., 110, 7557–7558.
Nirmala, N.R. and Wagner, G. (1989) J. Magn. Reson., 82, 659–661.
Orekhov, V.Y., Pervushin, K.V. and Arseniev, A.S. (1994) Eur. J. Biochem., 219, 887–896.
PalmerIII, A.G., Rance, M. and Wright, P.E. (1991) J. Am. Chem. Soc., 113, 4371–4380.
PalmerIII, A.G., Skelton, N.J., Chazin, W.J., Wright, P.E. and Rance, M. (1992) Mol. Phys., 75, 699–711.
PalmerIII, A.G., Hochstrasser, R.A., Millar, D.P., Rance, M. and Wright, P.E. (1993) J. Am. Chem. Soc., 115, 6333–6345.
Peng, J.W., Thanabal, V. and Wagner, G. (1991) J. Magn. Reson., 95, 421–427.
Peng, J.W. and Wagner, G. (1992a) J. Magn. Reson., 98, 308–332.
Peng, J.W. and Wagner, G. (1992b) Biochemistry, 31, 8571–8586.
Powers, R., Clore, G.M., Stahl, S.J., Wingfield, P.T. and Gronenborn, A. (1992) Biochemistry, 31, 9150–9157.
Press, W.H., Flannery, B.P., Teukolsky, S.A. and Vetterling, W.T. (1986) Numerical Recipes, Cambridge University Press, Cambridge.
Redfield, C., Boyd, J., Smith, L.J., Smith, R.A.G. and Dobson, C.M. (1992) Biochemistry, 31, 10431–10437.
Ribeiro, A.A., King, R., Restivo, C. and Jardetzky, O. (1980) J. Am. Chem. Soc., 102, 4040–4051.
Ringe, D. and Petsko, G.A. (1985) Prog. Biophys. Mol. Biol., 45, 197–235.
Schulz, G.E. and Schirmer, R.H. (1978) In Springer Advanced Texts in Chemistry, Vol. 1 (Ed., Cantor, C.R.) Springer, New York, NY, pp. 233–251.
Shaka, A.J., Keeler, J., Frenkiel, T. and Freeman, R. (1983) J. Magn. Reson., 52, 335–338.
Sklenář, V., Torchia, D. and Bax, A. (1987) J. Magn. Reson., 73, 375–379.
Solomon, I. (1955) Phys. Rev., 99, 559–565.
Stone, M.J., Fairbrother, W.J., PalmerIII, A.G., Reizer, J., Saier, M.H. and Wright, P.E. (1992) Biochemistry, 31, 4394–4406.
Stone, M.J., Chandrasekhar, K., Holmgren, A., Wright, P.E. and Dyson, H.J. (1993) Biochemistry, 32, 426–435.
Szyperski, T., Luginbühl, P., Otting, G., Güntert, P. and Wüthrich, K. (1993) J. Biomol. NMR, 3, 151–164.
Takashima, H., Amiya, S. and Tobayashi, Y. (1991) J. Biochem., 109, 807–810.
Teplyakov, A., Obmolova, G., Wilson, K. and Kuromizu, K. (1993) Eur. J. Biochem., 213, 737–741.
VanMierlo, C.P.M., Darby, N.J., Keeler, J., Neuhaus, D. and Creighton, T.E. (1993) J. Mol. Biol., 229, 1125–1146.
Wagner, G. and Wüthrich, K. (1986) Methods Enzymol., 131, 307–326.
Wagner, G. (1993) Curr. Opin. Struct. Biol., 3, 748–754.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mispelter, J., Lefèvre, C., Adjadj, É. et al. Internal motions of apo-neocarzinostatin as studied by 13C NMR methine relaxation at natural abundance. J Biomol NMR 5, 233–244 (1995). https://doi.org/10.1007/BF00211751
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00211751