Abstract
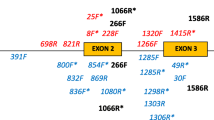
Major histocompatibility complex (Mhc) molecules bind self and foreign peptides and present them to lymphocyte for recognition. Activation of lympocytes by Mhc-bound foreign peptides leads to specific immune response against parasites. The Mhc genes have been studied extensively in mammals and birds but much less in other vertebrate classes. In this communication we provide the first description of the exon-intron organization of class II \-chain-encoding genes from the teleostfish Aulonocara hansbaenschi, family Cichlidae. Each of the genes consists of six exons, E1 through E6, encoding the leader peptide (E1), β domain (E1+E2), β domain (E3+E4), connecting peptide (E5), transmembrane region (E5), cytoplasmic domain (E5+E6), and the 3' untranslated region (E6). The exons are separated by relatively short introns, the length of the ongest intron being 1.3 kilobase pairs. An important difference between these and all other known class II β genes is that the \2 domain-encoding exon is split by an intron 97 base pairs in length. The intron is absent in other teleost fishes suchas Brachydanio rerio. A change in the 3' splice site of intron 4 in some of the genes of A. hansbaenschi and of another cichlid fish, Cypotilapia frontosa, has produced two extra codons at the 5' end of exon 5. Comparison of the A. hansbaenschi coding sequences with those of C. frontosa has revealed a concentration of variability in exon 2 and part of exon 3. Taken together, these observations provide evidence for the existence in cichlid fishes of at least two class I β loci which are functionally equivalent to the corresponding loci in mammals. The exon-intron organization and sequence similarities indicate that the two loci arose by duplication from a common ancestral gene.
Similar content being viewed by others
References
Brown, J. H., Jerdetzky, T.,Saper,. M. A., Samraoui, B., Bjorkman, OP. J., and Wiley, D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature 332: 845–850, 1988
Carson, S. and Trowsdale, J. Molecular organization of the class II genes of the human and mouse major histocompatibility complexes. Oxford Surv Eukaryot Genes 3: 63–94, 1986
Cosson, P., and Bonifacio, J. S. Role of transmembrane domain interactions in the assembly of class II MHC molecules. Science 258: 659–662, 1992
Dean, C., Pichersky, E., and Dunsmuir, P. Structure, evolution, and regulation of RbcS genes in higher plants. Annu Rev Plant Physiol Plant Mol Biol 40: 415–439, 1989
Feinberg, A. P. and Vogelstein, B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. AnalBiochem 132: 6–13, 1983
Flajnik, M. F., Canel, C., Kramer, J., and Kasahara, M. Evolution of the major histocompatibilty complex. Molecular cloning of major histocompatibility complex class I from the amphibian Xenopus. Proc Natl Acad Sci USA88: 537–541, 1991
Gorga, J. C. Structural analysisof class II major histocompatibility complex proteins. Crit Rev Immunol 11: 305–335, 1992
Grossberger, D. and Parham, P. Reptilian class I major histocompatibility complex genes reveal conserved elements in class I structure. Immunogenetics 36: 166–174, 1992
Hashimoto, K., Nakanishi, T., and Kurosawa, Y. Isolation of carp genes encoding major histocompatibility complexantigens. Proc Natl Acad Sci USA 87: 6863–6867, 1990
Hashimoto, K., Nakanishi, T., and Kurosawa, Y. Identification of a shark sequence resembling the major histocompatibility complex class I α3 domain. Proc Natl Acad Sci USA 89: 2209–2212, 1992
Holmes, D. S. and Quigley, M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem 114: 193–197, 1981
Hordvik, I., Grimholt, U., Fosse, V., Lie, Ø., and Endersen,C. Cloning and sequence analysis of cDNAs encoding the MHC class II β chain in Atlantic salmon, Salmo salar. Immunogenetics 37: 439–443, 1993
Hughes, A. L. and Nei, M. Pattern of nucleotide substitution at major histocompatibility complex class I roci reveals overdominant selection. Nature 335: 167–170, 1988
Juul-Madsen, H. R., Glamann, J., Madsen, H. O., and Simonsen, M. MHC class II beta-chain expression in the rainbow trout. Scand J Immunol 35: 687–694, 1992
Kasahara, M., Vasquez, M.,Sato, K., McKinney, E. C., and Flajnik, M. F. Evolution of the majorhistocompatibility complex: Isolation of class II A gene from the cartilaginous fish. Proc Natl Acad Sci USA 89: 6688–6692, 1992
Kelly, A. P., Monacco, J. J., Cho, S., and Trowsdale, J. A new human HLA class II-related locus, DM. Nature 353: 571–573, 1991
Klein, D., Ono, H., O'hUigin, C., Vincek, V., and Klein, J. Extensive Mhc variability in African cichlid fishes, Nature, Bubmitted, 1993
Klein, J. Natural History of the Major Histocompatibility Complex, John Wiley, New York, 1986
Klein, J. and Figueroa,F. Evolution of the major histocompatibility complex. CRC Crit Rev Immunol 6: 295–386, 1986
Klein, J., Bontrop, R. E.,Dawkins, R. L., Erlich, H. A., Gyllensten, U. B., Heise, E. R., Jones, P. P., Parham, P., Wakeland,E. K., and Watkins, D. I. Nomenclature for the major histocompatibility complexes of a different species: a proposal. Immunogenetics 31: 217–219, 1990
Klein, J., Satta, Y., O'hUigin, C., and Takahata, N. The molecular descent of the major histocompatibility complex. Annu Rev Immunol 11: 269–295, 1993
Nelson, J. S. Fishes of the World. JohnWiley, New York, 1984
Ono, H., Klein, D., Vincek, V., Figueroa, F., O'hUigin, C., Tichy, H., and Klein, J. Mhc class II genes of zebrafish. Proc Natl Acad Sci USA 89: 11886–11890, 1992
Ono, H., O'hUigin,C., Vincek, V., Stet, R. J. M., Figueroa, F., and Klein,J. New β chain-encoding Mhc class II genes in the carp. Immunogenetics 38: 146–149, 1993
Rothbard, J. B. and Gefter, M.L. Interactions between immunogenetic peptides and Mhc proteins. Annu Rev Immunol 9: 527–565, 1991
Saiki, R. K., Gelfland, D. H., Stoffel, S., Scharf, S. J., Higuchi, R., Horn, G. T., Mullis, K.B.,and Erlich, H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–491, 1988
Sanger, F., Nicklen, S.,and Coulson, A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467, 1977
Sato, K., Flajnik, M.F., Du Pasquier, L., Katagiri,M., and Kasahara, M. Evolution of the MHC: Isolation of class II \-chain cDNA clones from theamphibian Xenopus laevis. J Immunol. Submitted, 1992
Shapiro, M.B. and Senapathy, P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res 15: 7155–7174, 1987
Stressinger, G. Y., Okada, Y.,Emrich, J., Newton, J.,Tsugita, A., Terzaghi, E., and Inouye, M. Frameshift mutations and the genetic code. Cold Spring Harbor Quant Biol 31: 77–84, 1966
Ueyama, H., Hamada, H., Battula, N.,and Kakunaga, T. Structure of a human smooth muscle actin gene (aortic type) with a unique intron site. Mol Cell Biol 4: 1073–1078, 1984
Wu, T. T. and Kabat, E.A. An analysis of the sequences of the variable regions of Benco Jones proteins and myeloma light chains and their amplifications for antibody complementarity. J Exp Med 132: 211–250, 1970
Zhu, Z., Vincek, V., Figueroa, F., Schönbach, C., and Klein, J. Mhc-DRB genes of the pigtail macaque (Macaca nemestrina): implications for the evolution of human DRB genes. Mol Biol Evol 8: 563–578, 1991
Zoorob, R., Béhar, G., Kroemer, G., and Auffray, C. Organization of a functional chicken class II β gene. Immunogenetics 31: 179–187, 1990
Author information
Authors and Affiliations
Additional information
The nucleotide sequence date reported in this paper have been submitted to the GenBank nucleotide sequence database and have been assigned the accession numbers L13222-L13233.
Correspondence to: J. Klein
Rights and permissions
About this article
Cite this article
Ono, H., O'hUigin, C., Vincek, V. et al. Exon-intron organization of fish major histocompatibility complex class II B genes. Immunogenetics 38, 223–234 (1993). https://doi.org/10.1007/BF00211522
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00211522