Summary
Ultrastructural examination of the podium of the asteroid echinoderm Stylasterias forreri reveals that cells of the coelomic epithelium and cells of the retractor muscle are, in fact, components of a single epithelium. The basal lamina of this unified epithelium adjoins the connective tissue layer of the podium.
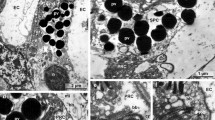
The principal epithelial cells in the coelomic lining are the flagellated adluminal cells and the myofilament-bearing retractor cells. Adluminal cells interdigitate extensively with each other and form zonular intermediate and septate junctions at their apicolateral surfaces. The adluminal cells emit processes which extend between the underlying retractor cells and terminate on the basal lamina of the epithelium. Retractor cells exhibit unregistered arrays of thick and thin myofilaments. The periphery of the retractor cell is characteristically thrown into keel-like folds which interdigitate with the processes of neighboring cells. Specialized intermediate junctions bind the retractor cells to each other and anchor the retractor cells to the basal lamina of the epithelium. The retractor cells are not surrounded by external laminae or connective tissue envelopes.
It is concluded that the coelomic lining in the podium of S. forreri is a bipartite epithelium and that the retractor cells of the podium are myoepithelial in nature. There are no detectable communicating (gap) junctions between the epithelial cells of the coelomic lining.
Similar content being viewed by others
Refrence
Atwood DG (1973) Ultrastructure of the gonadal wall of the sea cucumber, Leptosynapta clarki (Echinodermata: Holothuroidea). Z Zellforsch 141:319–330
Baccetti B, Rosati F (1968) The fine structure of the Polian vesicles of holothurians. Z Zellforsch 90:148–160
Bargmann W, Behrens B (1963) Über den Feinbau des Nervensystems des Seesterns (Asterias rubens L.). II. Mitteilung. Zur Frage des Baues und der Innervation der Ampullen. Z Zellforsch 59:746–770
Bargmann W, Behrens B (1964) Über die Tiedemannschen Organe des Seesterns (Asterias rubens L.). Z Zellforsch 63:120–133
Bargmann W, Harnack M, Jacob K (1962) Über den Feinbau des Nervensystems des Seesternes (Asterias rubens L.). I. Mitteilung. Ein Beitrag zur Vergleichenden Morphologie der Glia. Z Zellforsch 56:573–594
Buchanan JB (1962) A re-examination of the glandular elements in the tube feet of some common British ophiuroids. Proc Zool Soc London 138:645–650
Cavey MJ, Cloney RA (1972) Fine structure and differentiation of ascidian muscle. I. Differentiated caudal musculature of Distaplia occidentalis tadpoles. J Morphol 138:349–374
Cavey MJ, Wood RL (1979) Sarcoplasmic reticulum and sarcolemmal couplings in the podial muscle cells of an asteroid echinoderm. Am Zoologist 19:903
Cavey MJ, Wood RL (1981) Specializations for excitation-contraction coupling in the podial retractor cells of the starfish Stylasterias forreri. Cell Tissue Res 218:475–485
Cloney RA, Florey E (1968) Ultrastructure of cephalopod chromatophore organs. Z Zellforsch 89:250–280
Cobb JLS (1967) The innervation of the ampulla of the tube foot in the starfish Astropecten irregularis. Proc Roy Soc London Ser B 168:91–99
Cobb JLS, Raymond AM (1979) The basiepithelial nerve plexus of the viscera and coelom of eleutherozoan Echinodermata. Cell Tissue Res 202:155–163
Coleman R (1969a) Ultrastructure of the tube foot sucker of a regular echinoid, Diadema antillarum Philippi, with especial reference to secretory cells. Z Zellforsch 96:151–161
Coleman R (1969b) Ultrastructure of the tube foot wall of a regular echinoid, Diadema antillarum Philippi. Z Zellforsch 96:162–172
Dolder H (1972) Ultrastructural study of the smooth muscle in the tubefeet of the echinoderms, Asterina stellifera and Pentacta peterseni. J Submicr Cytol 4:221–232
Engster MS, Brown SC (1972) Histology and ultrastructure of the tube foot epithelium in the phanerozonian starfish, Astropecten. Tissue & Cell 4:503–518
Florey E, Cahill MA (1977) Ultrastructure of sea urchin tube feet. Evidence for connective tissue involvement in motor control. Cell Tissue Res 177:195–214
Green CR, Bergquist PR, Bullivant S (1979) An anastomosing septate junction in endothelial cells of the phylum Echinodermata. J Ultrastruct Res 68:72–80
Hamann O (1889) Anatomie und Histologie der Crinoiden. Jena Z Naturwiss 23:233–388
Holland ND (1971) The fine structure of the ovary of the feather star Nemaster rubiginosa (Echinodermata: Crinoidea). Tissue &Cell 3:161–175
Hyman LH (1955) The invertebrates, vol 4. McGraw-Hill Book Company, New York
Jensen H (1975) Ultrastructure of the dorsal hemal vessel in the sea-cucumber Parastichopus tremulus (Echinodermata: Holothuroidea). Cell Tissue Res 160:355–369
Kawaguti S (1964) Electron microscopic structures of the podial wall of an echinoid with special references to the nerve plexus and the muscle. Biol J Okayama Univ 10:1–12
Kawaguti S (1965) Electron microscopy on the ampulla of the echinoid. Biol J Okayama Univ 11:75–86
Kawaguti S, Kamishima Y (1964) Electron microscopic study on the integument of the echinoid, Diadema setosum. Annot Zool Japon 37:147–152
Kelley RO, Dekker RAF, Bluemink JG (1973) Ligand-mediated osmium binding: Its application in coating biological specimens for scanning electron microscopy. J Ultrastruct Res 45:254–258
Kelly DE, Shienvold FL (1976) The desmosome: Fine structural studies with freeze-fracture replication and tannic acid staining of sectioned epidermis. Cell Tissue Res 172:309–323
Lazarides E, Revel JP (1979) The molecular basis of cell movement. Scientific American 240:100–113
Luft JH (1961) Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol 9:409–414
Maximow AA (1930) A textbook of histology. Bloom W (ed) WB Saunders Company, Philadelphia
McNutt NS (1975) Ultrastructure of the myocardial sarcolemma. Circ Res 37:1–13
Menton DN, Eisen AZ (1970) The structure of the integument of the sea cucumber, Thyone briareus. J Morphol 131:17–36
Nichols D (1959a) The histology of the tube-feet and clavulae of Echinocardium cordatum. Quart J Microsc Sci 100:73–87
Nichols D (1959b) The histology and activities of the tube-feet of Echinocyamus pusillus. Quart J Microsc Sci 100:539–555
Nichols D (1960) The histology and activities of the tube-feet of Antedon bifida. Quart J Microsc Sci 101:105–117
Nichols D (1961) A comparative histological study of the tube-feet of two regular echinoids. Quart J Microsc Sci 102:157–180
Nichols D (1966) Functional morphology of the water-vascular system. In: Boolootian RA (ed) Physiology of Echinodermata. Interscience Publishers, New York
Nichols D (1967) Echinoderms. Hutchinson and Company, London
Nørrevang A, Wingstrand KG (1970) On the occurrence and structure of choanocyte-like cells in some echinoderms. Acta Zoologica Stockholm 51:249–270
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Richardson KC, Jarett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Schantz A, Scheeter A (1965) Iron-hematoxylin and safranin O as a polychrome stain for Epon sections. Stain Technol 40:279–282
Shienvold FL, Kelly DE (1976) The hemidesmosome: New fine structural features revealed by freezefracture techniques. Cell Tissue Res 172:289–307
Smith JE (1937) The structure and function of the tube feet in certain echinoderms. J Mar Biol Ass U K 22:345–357
Souza Santos H, Sasso WS (1968) Morphological and histochemical studies on the secretory glands of starfish tube feet. Acta Anat 69:41–51
Souza Santos H, Sasso WS (1970) Ultrastructural and histochemical studies on the epithelium revestment layer in the tube feet of the starfish Asterina stellifera. J Morphol 130:287–296
Walker CW (1979) Ultrastructure of the somatic portion of the gonads in asteroids, with emphasis on flagellated-collar cells and nutrient transport. J Morphol 162:127–162
Wood RL (1977) The cell junctions of hydra as viewed by freeze-fracture replication. J Ultrastruct Res 58:299–315
Wood RL, Cavey MJ (1980) Myoepithelial nature of podial retractor musculature in echinoderms. Am Zoologist 20:911
Wood RL, Kuda AM (1980) Formation of junctions in regenerating hydra: Septate junctions. J Ultrastruct Res 70:104–117
Wood RL, Luft JH (1965) The influence of buffer systems on fixation with osmium tetroxide. J Ultrastruct Res 12:22–45
Author information
Authors and Affiliations
Additional information
This investigation was supported by general research funds from the Department of Anatomy of the University of Southern California (R.L.W.) and by Research Operating Grant A0484 from the Natural Sciences and Engineering Research Council of Canada (M.J.C.). Ms. Aileen Kuda and Mr. Steve Osborne provided technical assistance. A portion of this study was conducted at the Friday Harbor Laboratories of the University of Washington, and the authors gratefully acknowledge the cooperation and hospitality of the Director, Dr. A.O. Dennis Willows
Rights and permissions
About this article
Cite this article
Wood, R.L., Cavey, M.J. Ultrastructure of the coelomic lining in the podium of the starfish Stylasterias forreri . Cell Tissue Res. 218, 449–473 (1981). https://doi.org/10.1007/BF00210107
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00210107