Abstract
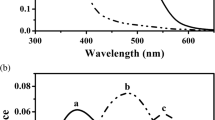
The goatfish Upeneus tragula undergoes an abrupt metamorphosis at settlement when the pelagic larvae begin a reef-associated benthic mode of life. A microspectrophotometric investigation of the retinal visual pigments was carried out on fish prior to, during, and following settlement. It was found that the visual pigment in the long wavelength-absorbing member of the double cones in the dorsal retina changed rapidly from a rhodopsin with a wavelength of maximum absorption (λmax) of 580 nm to that of 530 nm. The second member of the double cones always had a rhodopsin with the λmax absorbing at shorter wavelengths. Prior to settlement the average for this class of cones was 487 nm whereas during and immediately following the settlement period the λmax recorded from individual outer segments was found to vary between 480 nm and 520 nm, with two possible classes of cone absorbance emerging within this range. These two classes of absorbance had average λmax values of 487 and 515 nm. The average λmax of the paired cone classes in one larger wild-settled fish were found to be at 506 nm and 530 nm. No change was detected in the λmax of the single cones or the rods which were always found to have a λmax of about 400 nm and 498 nm respectively. The loss of the redabsorbing pigment occurred over the same time scale as the metamorphosis of morphological features associated with the settlement process. It is thought that the loss of this visual pigment is associated with the change in light environment of the fishes as they leave the surface waters to begin a benthic mode of life in deeper water.
Similar content being viewed by others
Abbreviations
- AIMS:
-
Australian Institute of Marine Science
- ANOVA:
-
Analysis of variance
- IR:
-
infra-red
- λmax:
-
wavelength of maximum absorption
- MSP:
-
microspectrophotometer
- NA:
-
numerical aperture
- SL:
-
standard length
References
Avery JA, Bowmaker JK (1982) Visual pigments in the four-eyed fish, Anableps anableps. Nature 298:62–63
Baker KS, Smith RC (1982) Bio-optical classification and model of natural waters. Limnol Oceanogr 27:500–509
Beatty DD (1984) Visual pigments and the labile scotopic visual system of fish. Vision Res 24:1563–1573
Blatz PE, Liebman PA (1973) Wavelength regulation in visual pigments. Exp Eye Res 17:573–580
Bok D, Young RW (1972) The renewal of diffusely distributed proteins in the outer segments of rods and cones. Vision Res 12:161–168
Bowmaker JK, Kunz YW (1987) Ultraviolet receptors, tetrachromatic colour vision and retinal mosaics in the brown trout (Salmo trutta): age-dependent changes. Vision Res 27:2101–2108
Carlisle DB, Denton EJ (1959) On the metamorphosis of the visual pigments of Anguilla anguilla. J Mar Biol Ass UK 38:97–102
Crescitelli F (1972) The visual cells and visual pigments of the vertebrate eye. In: Dartnall HJA (ed) Handbook of sensory physiology VII/1. Springer, Berlin Heidelberg New York, pp 245–365
Dartnall HJA, Lythgoe JN (1965) The spectral clustering of visual pigments. Vision Res 5:81–100
Jerlov NG (1976) Marine optics. Elsevier, Amsterdam
Kingsford MJ, Choat JH (1985) The fauna associated with drift algae captured with a plankton-mesh purse seine net. Limnol Oceanogr 30:618–630
Knowles A, Dartnall HJA (1977) The photobiology of vision. In: Davson H (ed) The eye, vol 2B. Academic Press, London, pp 1–689
Levine JS, MacNichol Jr EF (1979) Visual pigments in teleost fishes: effects of habitat, microhabitat, and behavior on visual system evolution. Sensory Process 3:95–131
Levine JS, MacNichol Jr EF (1985) Microspectrophotometry of primate photoreceptors: art, artefact, and analysis. In: Fein A, Levine JS (eds) The visual system. Liss, New York, pp 73–87
Loew ER, Dartnall HJA (1976) Vitamin A1/A2-based visual pigment mixtures in cones of the rudd. Vision Res 16:891–896
Loew ER, Lythgoe JN (1978) The ecology of cone pigments in teleost fishes. Vision Res 18:715–722
Loew ER, Wahl CM (1991) A short-wavelength sensitive cone mechanism in juvenile yellow perch, Perca flavescens. Vision Res 31:353–360
Lythgoe JN (1984) Visual pigments and environmental light. Vision Res 24:1539–1550
Lythgoe JN, Partridge JC (1989) Visual pigments and the acquisition of visual information. J Exp Biol 146:1–20
McCormick MI (1992) The influence of pelagic life history on the quality of tropical goatfish (Family Mullidae) at settlement. PhD Thesis, James Cook University of North Queensland
McCormick MI (1993) Development and change at settlement in the barbel structure of the reef fish, Upeneus tragula (Family: Mullidae). Environ Biol Fish (in press)
McFarland WN (1986) Light in the sea — correlations with behaviours of fishes and invertebrates. Am Zool 26:389–401
McFarland WN, Munz FW (1975) The photic environment of clear tropical seas during the day. Vision Res 15:1063–1070
Muntz WRA, Mouat GVS (1984) Annual variations in the visual pigments of brown trout inhabiting lochs providing different light environments. Vision Res 24:1575–1580
Munz FW, McFarland WN (1973) The significance of spectral position in the rhodopsins of tropical marine fishes. Vision Res 13:1829–1874
Nathans J, Thomas D, Hogness DS (1986) Molecular genetics of human color vision: the genes encoding blue, green and red pigments. Science 232:193–202
O'Day WT, Young RW (1978) Rhythmic shedding of outersegment membranes by visual cells in goldfish. J Cell Biol 76:593–604
Partridge JC, De Grip WJ (1991) A new template for rhodopsin (vitamin A1 based) visual pigments. Vision Res 31:619–630
Partridge JC, Speare P, Shand J, Muntz WRA, Williams DMcB (1992) Microspectrophotometric determinations of rod visual pigments in some adult and larval Australian amphibians. Visual Neurosci 9:137–142
Shand J (1993) Changes in retinal structure during development and settlement of the goatfish Upeneus tragula. Brain Behav Evol (in press)
Shand J, Partridge JC, Archer SN, Potts GW, Lythgoe JN (1988) Spectral absorbance changes in the violet/blue sensitive cones of the juvenile pollack, Pollachius pollachius. J Comp Physiol A 163:699–703
Victor BC (1991) Settlement strategies and biogeography of reef fishes. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic Press, San Diego, pp 231–260
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shand, J. Changes in the spectral absorption of cone visual pigments during the settlement of the goatfish Upeneus tragula: the loss of red sensitivity as a benthic existence begins. J Comp Physiol A 173, 115–121 (1993). https://doi.org/10.1007/BF00209623
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00209623