Abstract
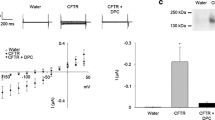
Mutations in the AE1 (band 3) anion exchanger of human erythrocytes have been associated with altered red cell shape and heritable disease.The Southeast Asian Ovalocytosis (SAO) AE1 mutation, a 27 nt deletion producing the Δ400–408 form of AE1, and the AE1 Prague mutation, a 10 nt insertion producing a frameshift after AE1 aa 821 leading to premature termination, are found only in the heterozygous state. We therefore examined accumulation and function of wt AE1 polypeptide in Xenopus oocytes when coexpressed with AE1 SAO and with AE1 Prague. Our SAO construct lacked the K56E (AE1 Memphis) polymorphism present in the endogenous AE1 SAO protein. Neither mutant AE1 mediated Cl−} uptake into cRNA-injected Xenopus oocytes. Coninjection of mutant and wt cRNAs led to dose-dependent inhibition of wt function by AE1 Prague, but not by SAO. Though in vitro translation of the two mutants revealed little difference in their insertion into microsomal membranes, AE1 Prague accumulated in Xenopus oocytes to lower levels than did AE1 SAO or wt. Unlike AE1 SAO polypeptide, AE1 Prague polypeptide was not detectable at the oocyte surface. Moreover, overexpression of AE1 Prague, in contrast to AE1 SAO, reduced the accumulation of wt AE1, at the oocyte surface. This inhibition occurred in the absence of detectable heteromer formation between the AE1 Prague and wt AE1 polypeptides.
Similar content being viewed by others
References
Drumm, M.L., Wilkinson, D.J., Smit, L.S., Worrell, R.T., Strong, T.V., Frizzell, R.A., Dawson, D.C., Collins, P.C. 1991. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science 254:1797–1799
Garcia, A.-M., Lodish, H.F. 1989. Lysine 539 of human band 3 is not essential for ion transport or inhibition by stilbene disulfonates. J. Biol. Chem. 264:19607–19613
Groves, J.D., Ring, S.M., Schofield, A.E., Tanner, M.J.A. 1992. The expression of the abnormal human red cell anion transporter from South-East Asian ovalocytes (band 3 SAO) in Xenopus oocytes. FEBS Lett. 330:186–190
Groves, J.D., Tanner, M.J.A. 1993. The effects of glycophorin A on the expression of the human red cell anion transporter (band 3) in Xenopus oocytes. J. Membrane Biol. 140:81–88
Groves, J.D., Tanner, M.J.A. 1993. Role of N-glycosylation in the expression of human band 3-mediated anion transport. Mol. Membr. Biol. 11:31–38
Humphreys, B.D., Jiang, L., Chernova, M., Alper, S.L. 1994. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol (Cell) 266:C1295-C1307
Husain-Chishti, A., Andrabi, K., Palek, J., Amato, D., Liu, S.C. 1991. Altered tyrosine phosphorylation of the red cell band 3 protein in malaria-resistant southeast asian ovalocytosis (SAO). Blood 78:80a
Jarolim, P., Palek, J., Amato, D., Hassan, K., Sapak, P., Nurse, G.T., Rubin, H.L., Zhai, S., Sahr, K.E., Liu, S.-C. 1991. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc. Natl. Acad. Sci. USA 88:11022–11026
Jarolim, P., Rubin, H.L., Zhai, S., Sahr, K.E., Liu, S.-C., Mueller, T.J., Palek, J. Band 3 Memphis: a widespread polymorphism with abnormal electrophoretic mobility of erythrocyte band 3 protein caused by substitution AAG-GAG (Lys-Glu) in codon 56. 1992. Blood 80:1592–1598
Jarolim, P., Rubin, H., Liu, S.-C., Cho, M., Brabec, V., Derrick, L., Yi, S., Saad, S., Alper, S., Brugnara, C., Golan, D., Palek, J. 1994. Band 3 Prague: a frameshift duplication in the erythroid band 3 gene in a kindred with hereditary spherocytosis with band 3 protein deficiency. J. Clin. Invest. 93:121–130
Ideguchi, H., Okubo, K., Ishikawa, A., Futata, Y., Hamasaki, N. 1992. Band 3-Memphis is associated with a lower transport rate of phosphoenolpyruvate. Br. J. Hematol. 82:122–125
Jennings, M.L., Gosselink, P.G. 1995. Anion exchange protein of Southeast Asian Ovalocytes: heterodimer formation between normal and variant subunits. Biochemistry (in press)
Jones, G.L., Edmundson, H.M., Wesche, D., Saul, A. 1991. Human erythrocyte Band-3 has an altered N terminus in malaria-resistant Melanesian ovalocytosis. Biochim. Biophys. Acta 1096:33–40
Komuro I., Wenninger, K.E., Phillipson, K.M., Izumo, S., 1992. Molecular cloning and characterization of the human cardiac Na+/ Ca2+ exchanger cDNA. Proc. Natl. Acad. Sci. USA 89:4769–4773
Liu, S.-C., Zhai, S., Palek, J., Golan, D.E., Amato, D., Hassan, K., Nurse, G.T., Babona, D., Coetzer, T., Jarolim, P., Zaik, M., Borwein, S. 1990. Molecular defect of the band 3 protein in Southeast Asian Ovalocytosis. New Eng. J. Med. 323:1530–1538
Liu, S.-C., Jarolim, P., Rubin, H.L., Palek, J., Amato, D., Hassan, K., Zaik, M., Sapak, P. 1994. The homozygous state for the band 3 protein mutation in Southeast Asian Ovalocytosis may be lethal. Blood 84:3590–3593
Liu, S.-C., Palek, J., Scott, J.Y., Nichols, P.E., Derick, L.H., Chiou, S.-S., Amato, D., Corbett, J.D., Cho, M.R., Golan, D.E. 1995. Molecular basis of altered red cell membrane properties in Southeast Asian Ovalocytosis: role of the mutant band 3 protein in band 3 oligomerization and retention by the membrane skeleton. Blood (in press)
Lux, S.E., John, K.M., Kopito, R.R., Lodish, H.F. 1989. Cloning and characterization of band 3, the human erythrocyte anionexchange protein (AE1). Proc. Natl. Acad. Sci. USA 86:9089–9093
MacKinnon, R. 1992. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature 350:232–235
Mohandas, N., Winardi, R., Knowles, D., Leung, A., Parra, M., George, E., Conboy, J., Chasis, J. 1992. Molecular basis for membrane rigidity of hereditary ovalocytosis. J. Clin. Invest. 89:686–692
Moriyama, R., Ideguchi, H., Lombarde, C.R., Van Dort, H.M., Low, P.S. 1992. Structural and functional characterization of band 3 from Southeast Asian ovalocytes. J. Biol. Chem. 267:25792–15797
Reithmeier, R.A.F., Casey, J.R. 1992. Oligomeric structure of the human erythrocyte band 3 anion transport protein. Prog. Cell Res. 2:181–190
Sarabia, V.E., Casey, J.R., Reithmeier, R.A.F. 1993. Molecular characterization of the band 3 protein from Southeast Asian ovalocytes. J. Biol. Chem. 268:10676–10680
Schofield, A.E., Reardon, D.M., Tanner, M.J.A. 1992. Defective anion transport activity of the abnormal band 3 in hereditary ovalocytic red blood cells. Nature 355:836–838
Schofield, A.E., Tanner, M.J.A., Pinder, J.C., Clough, B., Bayley, P.M., Nash, G.B., Dluzewski, A.R., Reardon, D., Cox, T., Wilson, R.J.M., Gratzer, W.B. 1992. Basis of unique red cell membrane properties in hereditary ovalocytosis. J. Mol. Biol. 223:949–958
Steinmeyer, K., Lorenz, C., Pusch, M., Koch, M.C., Jentsch, T.J. 1994. Multimeric structure of C1C-1 chloride channel revealed by mutations in dominant myotonia congenita (Thomsen). The EMBO J. 13:737–741
Tilley, L., McPherson, R.A., Jones, G.L., Sawyer, W.H. 1993. Structural organization of band 3 in Melanesian ovalocytes. Biochim. Biophys. Acta. 181:83–89
Yannoukakos, D., Vasseur, C., Driancourt, C., Blouquit, Y., Delaunay, J., Wajcman, H., Bursaux, E. 1991. Human erythrocyte band 3 polymorphism (Band 3 Memphis): Characterization of the structural modification (Lys 56-Glu) by protein chemistry methods. Blood 78:1117–1120
Author information
Authors and Affiliations
Additional information
We thank H. Rubin for technical assistance, I. Komuro and S. Izumo for the NCE1 cDNA, Y. Zhang for the AE11-422 cRNA; and A.M. Garcia for antibody to human AE1 and for comments on the manuscript. This work was supported by NIH grants DK-43495 (to S. Alper) and HL-37462 and HL-27215 (to J. Palek). S. Alper is an Established Investigator of the American Heart Association.
Rights and permissions
About this article
Cite this article
Chernova, M.N., Jarolim, P., Palek, J. et al. Overexpression of AE1 Prague, but not of AE1 SAO, inhibits wild-type AE1 trafficking in Xenopus oocytes. J. Membarin Biol. 148, 203–210 (1995). https://doi.org/10.1007/BF00207276
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00207276