Summary
The coupling mechanism between the bilaterally paired optic lobe circadian pacemakers in the cricket Gryllus bimaculatus was investigated by recording locomotor activity, under constant light or constant red light, after the optic nerve was unilaterally severed.
-
1.
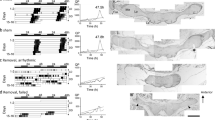
The majority (about 70%) of the animals showed a locomotor rhythm with 2 rhythmic components; one freerunning with a period of 25.33 ± 0.41 (SD) h and the other with 24.36 ± 0.37 (SD) h under constant light (Fig. 3A).
-
2.
Removal of the intact side optic lobe abolished the longer period component (Fig. 4A), while the operation on the operated side caused a reverse effect (Fig. 4B), indicating that the longer and the shorter period components are driven by the pacemaker on the intact and the operated side, respectively.
-
3.
The activity driven by a pacemaker was inhibited during the subjective day of the contralateral pacemaker (circadian time 0–10, Fig. 5).
-
4.
The freerunning periods of the two components were not constant but varied as a function of the mutual phase angle relationship (Figs. 3A, 7, 8).
These results suggest that the 2 optic lobe pacemakers weakly couple to one another and that the cricket maintains a stable temporal structure in its behavior through the phase-dependent mututal inhibition of activity and the phase-dependent freerunning period modulation.
Similar content being viewed by others
Abbreviations
- CT :
-
circadian time
- DD :
-
constant darkness
- LD :
-
light-dark cycle
- LL :
-
constant light
- RR :
-
constant red light
References
Block GD, Roberts MH (1981) Circadian pacemaker in the Bursatella eye: properties of the rhythm and its effect on locomotor behavior. J Comp Physiol 142:403–410
Boulus Z, Terman M (1979) Splitting of circadian rhythm in the rat. J Comp Physiol 134:75–83
Eskin A (1979) Introduction to symposium “Identification and physiology of circadian pacemakers.” Fed Proc 38:2570–2572
Eskin A, Harcombe E (1977) Eye of Navanax: optic activity, circadian rhythm and morphology. Comp Biochem Physiol 57A:443–449
Fleissner G (1982) Isolation of an insect circadian clock. J Comp Physiol 149:311–316
Honegger H-W, Schürmann FW (1975) Cobalt sulphide staining of optic fibres in the brain of the cricket, Gryllus campestris. Cell Tissue Res 159:213–225
Inouye ST, Kawamura H (1979) Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA 76:5962–5966
Jacklet JW (1969) Circadian rhythm of optic nerve impulses recorded in darkness from isolated eye of Aplysia. Science 164:562–563
Koehler WK, Fleissner G (1978) Internal desynchronization of bilaterally organized oscillators in the visual system of insects. Nature 273:708–710
Kronauer RE, Czeisler CA, Pilato SF, Moore-Ede MC, Weitzman ED (1982) Mathematical model of the human circadian system with two interacting oscillators. Am J Physiol 242:R3-R17
Loher W (1972) Circadian control of stridulation in the cricket, Teleogryllus commodus Walker. J Comp Physiol 79:179–190
Nishiitsutsuji-Uwo J, Pittendrigh CS (1968) Central nervous system control of circadian rhythmicity in cockroach. III. The optic lobes, locus of the driving oscillation? Z Vergl Physiol 58:14–46
Okada Y, Tomioka K, Chiba Y (1991) Circadian phase response curves for light in nymphal and adult crickets, Gryllus bimaculatus. J Insect Physiol (in press)
Page TL (1978) Interactions between bilaterally paired components of the cockroach circadian system. J Comp Physiol 124:225–236
Page TL (1982) Transplantation of the cockroach circadian pacemaker. Science 216:73–75
Page TL (1983) Effects of optic-tract regeneration on internal coupling in the circadian system of the cockroach. J Comp Physiol 153:353–363
Page TL (1987) Serotonin phase-shifts the circadian rhythm of locomotor activity in the cockroach. J Biol Rhythms 2:23–34
Page TL, Caldarola PC, Pittendrigh CS (1977) Mutual entrainment of bilaterally distributed circadian pacemakers. Proc Natl Acad Sci USA 74:1277–1281
Pickard GE, Turek FW (1982) Splitting of the circadian rhythm of activity is abolished by unilateral lesions of the suprachiasmatic nuclei. Science 215:1119–1121
Roberts MH, Block GD, Lusska AE (1987) Comparative studies of circadian pacemaker coupling in opisthobranch molluscs. Brain Res 423:286–292
Sato T, Kawamura H (1984) Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutamias sibiricus). Neurosci Res 1:45–52
Tomioka K, Chiba Y (1982a) Persistence of circadian ERG rhythm in the cricket with optic tract severed. Naturwissenschaften 69:355–356
Tomioka K, Chiba Y (1982b) Post-embryonic development of circadian rhythm in the cricket, Gryllus bimaculatus: a rhythm reversal. J Comp Physiol 147:299–304
Tomioka K, Chiba Y (1984) Effects of nymphal stage optic nerve severance or optic lobe removal on the circadian locomotor rhythm of the cricket, Gryllus bimaculatus. Zool Sci 1:385–394
Tomioka K, Chiba Y (1986) Circadian rhythm in the neurally isolated lamina-medulla complex of the cricket, Gryllus bimaculatus. J Insect Physiol 32:747–755
Tomioka K, Chiba Y (1989) Photoperiodic entrainment of locomotor activity in crickets (Gryllus bimaculatus) lacking the optic lobe pacemaker. J Insect Physiol 35:827–835
Tomioka K, Chiba Y (1991) Mutual communication between optic lobe pacemakers and constant light affect developing circadian system in the cricket, Gryllus bimaculatus. (submitted)
Tomioka K, Okada Y, Chiba Y (1990) Distribution of circadian photoreceptors in the compound eye of the cricket Gryllus bimaculatus. J Biol Rhythms 5:303–313
Waddel B, Lewis RD, Engelmann W (1990) Localization of the circadian pacemakers of Hemideina thoracica (Orthoptera; Stenopelmatidae). J Biol Rhythms 5:131–139
Wiedenmann G (1983) Splitting in a circadian activity rhythm: the expression of bilaterally paired oscillators. J Comp Physiol 150:51–60
Wiedenmann G, Krueger-Alef K, Martin W (1988) The circadian control of calling song and walking activity patterns in male crickets (Teleogryllus commodus). Exp Biol 47:127–137
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tomioka, K., Yamada, K., Yokoyama, S. et al. Mutual interactions between optic lobe circadian pacemakers in the cricket Gryllus bimaculatus . J Comp Physiol A 169, 291–298 (1991). https://doi.org/10.1007/BF00206993
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00206993