Abstract
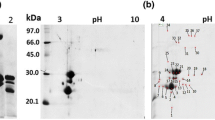
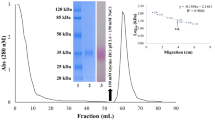
Proteins from Lupinus albus L. cv. Rio Maior seeds were fractionated according to solubility criteria. Patterns of concanavalin A (ConA)-binding polypeptides from the different classes, albumins, globulins, glutelins and prolamins, were established by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Two bands of apparent molecular masses of 29 and 23.5 kDa with glutelin solubility characteristics bound the lectin. The 23.5-kDa band was separated by two-dimensional electrophoresis into two components: one glycosylated and heterogeneous with an isoelectric point of approx. 10 (designated as G23) and another, not detected with ConA, precipitating in the first dimension. The amino acid and hexosamine analysis of G23 showed that it is particularly rich in Gly (11.2%), Glx (10.0%), Ser (9.0%), Leu (8.2%), Asx (7.5%), and Pro (6.7%) and that it has a considerable content of the sulphur-containing amino acids Met (2.0%) and Cys (5.8%) and contains glucosamine. The determined N-terminal amino acid sequence of G23 was: 1KG(R)V5KGTGD10(T)PXXV15XLY(N)R20T, and this had no significant similarity to any of the amino acid sequences contained in the data bank SWISS-PROT 26. The glycoprotein G23 was completely deglycosylated with peptide-N-glycosidase F, yielding a homogeneous 21-kDa polypeptide composed of approximately 191 amino acids. The structures of the major N-linked neutral oligosaccharides of G23, determined by exoglycosidase sequencing, were as follows: Manα2Manα6(Manα3) Manα6(Manα2Manα2Manα3)Manβ4GlcNAcβ4GlcNAc (13%); ± Manα2Manα6(Manα3)Manα6(± Manα2 Manα2 Manα3)Manβ4GlcNAcβ4GlcNAc (29%); Manα6(Manα3) Manα6(Manα2Manα3)Manβ4GlcNAcβ4GlcNAc (13%); Manα6(Manα3)Manα6(Manα3)Manβ4GlcNAcβ4GlcNAc (16%); Manα6(Manα3)(Xylβ2)Manβ4GlcNAc β4GlcNAc (28%). Changes in G23 abundance during seed development, germination and seedling growth were monitored with a specific antibody. The glycoprotein G23 started to accumulate appreciably during seed formation between the 40th and the 50th days after anthesis and was detected following seed imbibition, until the 9th day in cotyledons, the 2nd day in roots and the 4th day in hypocotyls and leaves.
Similar content being viewed by others
Abbreviations
- ConA:
-
concanavalin A
- Endo H:
-
endo-N-acetyl-β-d-glucosaminidase H
- GlcNAc:
-
N-acetylglucosamine
- gu:
-
glucose unit
- IEF:
-
isoelectric focusing
- Man:
-
mannose
- NEPHGE:
-
non-equilibrium pH gradient electrophoresis
- PNGase F:
-
peptide-N-glycosidase F
- PVDF:
-
polyvinylidenedifluoride
- Xyl:
-
xylose
References
Ashford D, Dwek RA, Welply JK, Amatayakul S, Homans SW, Lis H, Taylor GN, Sharon N, Rademacher TW (1987) The β1 → 2-d-xylose and α1 → 3-l-fucose substituted N-linked oligosaccharides from Erythrina cristagalli lectin. Eur J Biochem 166: 311–320
Ashford DA, Dwek RA, Rademacher TW, Lis H, Sharon N (1991) The glycosylation of glycoprotein lectins. Intra- and intergenus variation in N-linked oligosaccharide expression. Carbohydr Res 213: 215–227
Bairoch A, Boeckmann B (1991) The SWISS-PROT protein sequence data bank. Nucleic Acids Res 19 Suppl: 2247–2248
Bensadoun A, Weinstein D (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70: 241–250
Butters, TD, Scudder P, Rotsaert J, Petursson S, Fleet GWJ, Willenbrock FW, Jacobs GS (1991) Purification to homogeneity of Charonia lampas α-fucosidase by using sequential ligand-affinity chromatography. Biochem J 279: 189–195
Casey R, Domoney C, Ellis N (1986) Legume storage proteins and their genes. Oxford Surveys Plant Mol Cell Biol 3: 1–95
Cerletti P, Fumagalli A, Venturin D (1978) Protein composition of seeds of Lupinus albus. J Food Sci 43: 1409–1411
Costa J, Ricardo CPP (1994) Changes in the composition of ConA-binding glycopeptides from Lupinus albus root membranes induced by nodulation and callus formation. Plant Physiol Biochem 32: 295–302
Duranti M, Restani P, Poniatowska M, Cerletti P (1981) The seed globulins of Lupinus albus. Phytochemistry 20: 2071–2075
Duranti M, Guerrieri N, Cerletti P, Vecchio G (1992) The legumin precursor from white lupin seed. Eur J Biochem 206: 941–947
Faye L, Chrispeels MJ (1985) Characterization of N-linked oligosaccharides by affinoblotting with concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal Biochem 149: 218–224
Faye L, Chrispeels MJ (1989) Apparent inhibition of β-fructosidase secretion by tunicamycin may be explained by breakdown of the unglycosylated protein during secretion. Plant Physiol 89: 845–851
Higushi W, Fukasawa C (1987) A rice glutelin and a soybean glycinin have evolved from a common ancestral gene. Gene 55: 245–253
Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81: 802–806
Krebbers E, van Rompary J, Vandekerckhove J (1993) Expression of modified seed storage proteins in transgenic plants. In: Hiatt A (ed) Transgenic plants: fundamentals and applications. Marcel Dekker, New York, pp 37–60
Krishnan HB, Okita TW (1986) Structural relationship among the rice glutelin polypeptides. Plant Physiol 81: 748–753
Krishnan HB, Pueppke SG (1993) Nucleotide sequence of an abundant rice seed globulin: homology with the high molecular mass glutelins of wheat, rye and triticale. Biochem Biophys Res Com 193: 460–466
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Lamport DTA (1980) Structure and function of plant glycoproteins. In: Preiss J (ed) The biochemistry of plants. Academic Press, New York, pp 501–541
Li YT, Li SC (1972) α-Mannosidase, β-N-acetylhexosaminidase and β-galactosidase from Jack bean meal. Methods Enzymol 28: 702–713
Lipman DJ, Pearson WR (1985) Rapid and sensitive protein similarity searches. Science 227: 1435–1441
McManus MT, Mckeating J, Secher DS, Osborne DJ, Ashford D, Dwek RA, Rademacher TW (1988) Identification of a monoclonal antibody to abscission tissue that recognizes xylose/fucose-containing N-linked oligosaccharides from higher plants. Planta 175: 506–512
Montreuil J, Bouquelet S, Debray H, Fournet B, Spit G, Strecker G (1986) Glycoproteins. In: Chaplin MF, Kennedy JF (eds) Carbohydrate analysis. IRL Press, Oxford, pp 143–204
O'Farrell PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250: 4007–4021
O'Farrell PZ, Goodman HM, O'Farrell PH (1977) High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12: 1133–1142
Okita TW, Hwang YS, Hnilo J, Kim WT, Aryan AP, Larson R, Krishnan HB (1989) Structure and expression of the rice glutelin multigene family. J Biol Chem 264: 12573–12581
Osborne TB (1924) The vegetable proteins. Longmans Green, London
Parekh RB, Dwek RA, Thomas JR, Opdenakker G, Rademacher TW, Wittwer AJ, Howard SC, Nelson R, Siegel NR, Jennings MG, Harakas NK, Feder J (1989) Cell-type specific and site-specific N-glycosylation of type I and type II human tissue plasminogen activator. Biochemistry 28: 7644–7662
Righetti PG (1983) General experimental aspects. In: Work TS, Burdon RH (eds) Isoelectric focusing: theory, methodology and applications. Elsevier Biomedical Press, Amsterdam, pp 299–303
Sturm A, Van Kuik JA, Vliegenthart JFG, Chrispeels MJ (1987) Structure, position, and biosynthesis of the high mannose and the complex oligosaccharide side chains of the bean storage protein phaseolin. J Biol Chem 262: 13392–13403
Takahashi N, Hotta T, Ishihara H, Mori M, Tejima S, Bligny R, Akazawa T, Endo S, Arata Y (1986) Xylose-containing common structural unit in N-linked oligosaccharides of laccase from sycamore cells. Biochemistry 25: 388–395
Tarr GE (1986) Manual Edman sequencing system. In: Shively JE (ed) Methods of protein microcharacterisation. Human Press, Clifton, NJ, USA, pp 155–194
Tovey ER, Baldo BA (1989) Specialized aspects of protein blotting. In: Baldo BA, Tovey ER (eds) Protein blotting. St. Leonards, NSW, Karger, pp 43–64
Towbin H, Staehlin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Wen TN, Luthe DS (1985) Biochemical characterization of rice glutelin. Plant Physiol 78: 172–177
Woodward MP, Young WW, Jr Bloodgood RA (1985) Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods 78: 143–153
Yamagata H, Sugimoto T, Tanaka K, Kasai Z (1982) Biosynthesis of storage proteins in developing rice seeds. Plant Physiol 70: 1094–1100
Zhao WM, Gatehouse JA, Boulter D (1983) The purification and partial amino acid sequence of a polypeptide from the glutelin fraction of rice grains; homology to pea legumin. FEBS Lett 162: 96–102
Author information
Authors and Affiliations
Additional information
We thank Geoffrey Guile (Oxford Glycobiology Institute, Oxford, UK) for help with HPLC separations and amino acid and hexosamine analysis, Terry Butters (Oxford Glycobiology Institute) for providing the exoglycosidases and advice in their use, Manuela Regala (Instituto de Tecnologia Química e Biológica Oeiras, Portugal) and Paula Veríssimo (University of Coimbra, Portugal) for determining the N-terminal amino acid sequence of G20 and G23 and Dr. Jorge Lampreia (Universidade Nova de Lisboa, Lisbon, Portugal) for the computerised search of the SWISS-PROT data bank. Lupinus albus seeds were provided by Dr. João Neves Martins (Instituto Superior de Agronomia Lisbon, Portugal). We also thank J. Romão (Instituto Gulbenkian de Ciência, Oeiras, Portugal) for technical assistance in antibody production. This work was supported by Junta National de Investigação Científica e Tecnológica, Portugal.
Rights and permissions
About this article
Cite this article
Costa, J., Ashford, D.A. & Pinto Ricardo, C.P. Characterisation of a developmentally related polypeptide with glutelin solubility characteristics from Lupinus albus L.. Planta 198, 221–229 (1996). https://doi.org/10.1007/BF00206247
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00206247