Summary
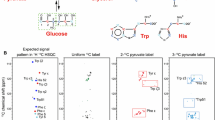
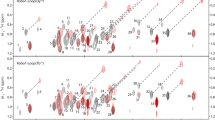
Three-dimensional 1H-TOCSY-relayed ct-[13C,1H]-HMQC is a novel experiment for aromatic spin system identification in uniformly 13C-labeled proteins, which is implemented so that it correlates the chemical shift of a given aromatic proton with those of the directly attached carbon and all vicinal protons. The ct-HMQC scheme is used both for overlay of the indirect 1H and 13C chemical shift evolution periods and for the generation of 1H-1H antiphase magnetization to accelerate the 1H-TOCSY magnetization transfer at short mixing times. As an illustration, data recorded for the 18 kDa protein cyclophilin A are presented. Since transverse relaxation of 13C-1H zero-quantum and double-quantum coherences is to first order insensitive to 13C-1H heteronuclear dipolar relaxation, the new experiment should work also for proteins with molecular weights above 20 kDa.
Similar content being viewed by others
References
Archer, S.J., Vinson, V.K., Pollard, T.D. and Torchia, D.A. (1993) Biochemistry, 32, 6680–6687.
Bax, A., Griffey, R.H. and Hawkins, B.L. (1983) J. Magn. Reson., 55, 301–315.
Bax, A., Ikura, M., Torchia, D.A., Kay, L.E. and Tschudin, R. (1990) J. Magn. Reson., 86, 304–318.
Bax, A. and Pochapsky, S.S. (1992) J. Magn. Reson., 99, 638–643.
Bax, A. and Grzesiek, S. (1993) Acc. Chem. Res., 26, 131–138.
Bodenhausen, G. and Ruben, D.J. (1980) Chem. Phys. Lett., 69, 185–189.
Braunschweiler, L. and Ernst, R.R. (1983) J. Magn. Reson., 53, 521–528.
Damberger, F.F., Pelton, J.G., Harrison, C.J., Nelson, H.C.M. and Wemmer, D.E. (1994) Protein Sci., 3, 1806–1821.
DeMarco, A. and Wüthrich, K. (1976) J. Magn. Reson., 24, 201–204.
Griesinger, C., Otting, G., Wüthrich, K. and Ernst, R.R. (1988) J. Am. Chem. Soc., 110, 7870–7872.
Griffey, R.H. and Redfield, A.G. (1987) Q. Rev. Biophys., 19, 51–82.
Grzesiek, S. and Bax, A. (1995a) J. Am. Chem. Soc., 117, 6527–6531.
Grzesiek, S. and Bax, A. (1995b) J. Biomol. NMR, 6, 335–339.
Grzesiek, S., Kuboniwa, H., Hinck, A.P. and Bax, A. (1955) J. Am. Chem. Soc., 117, 5312–5315.
Güntert, P., Dötsch, V., Wider, G. and Wüthrich, K. (1992) J. Biomol. NMR, 2, 619–629.
Hansen, P.E. (1981) Prog. NMR Spectrosc., 14, 175–296.
Horak, R.M., Steyn, P.S. and Vleggaar, R. (1985) Magn. Reson. Chem., 23, 995–1039.
Ikura, M., Kay, L.E., Tschudin, R. and Bax, A. (1990) J. Magn. Reson., 86, 204–209.
Ikura, M., Kay, L.E. and Bax, A. (1991) J. Biomol. NMR, 1, 299–304.
Kay, L.E., Wittekind, M., McCoy, M.A., Friedrichs, M.S. and Müller, L. (1992) J. Magn. Reson., 98, 443–450.
Krivdin, L.B. and Kalabin, G.A. (1989) Prog. NMR Spectrosc., 21, 293–448.
Levitt, M.H., Freeman, R. and Frenkiel, T. (1982) J. Magn. Reson., 75, 328–330.
Logan, T.M., Zhou, M.M., Nettesheim, D.G., Meadows, R.P., Van, Etten, R.L. and Fesik, S.W. (1994) Biochemistry, 33, 11087–11096.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989) J. Magn. Reson., 85, 393–399.
Müller, L. (1979) J. Am. Chem. Soc., 101, 4481–4484.
Otting, G. and Wüthrich, K. (1990) Q. Rev. Biophys., 23, 39–96.
Rance, M., Bodenhausen, G., Wagner, G., Wüthrich, K. and Ernst, R.R. (1985) J. Magn. Reson., 62, 497–510.
Seip, S., Balbach, J. and Kessler, H. (1992) J. Magn. Reson., 100, 406–410.
Shaka, A.J., Lee, C.J. and Pines, A. (1985) J. Magn. Reson., 54, 547–552.
Shaka, A.J., Lee, C.J. and Pines, A. (1988) J. Magn. Reson., 77, 274–293.
Sørensen, O.W., Eich, G.W., Levitt, M.H., Bodenhausen, G. and Ernst, R.R. (1983) Prog. NMR Spectrosc., 16, 163–192.
Sørensen, O.W., Rance, M. and Ernst, R.R. (1984) J. Magn. Reson. 56, 527–534.
Spitzfaden, C., Braun, W., Wider, G., Widmer, H. and Wüthrich, K. (1994) J. Biomol. NMR, 4, 463–482.
Subramanian, S. and Bax, A. (1987) J. Magn. Reson., 71, 325–330.
Szyperski, T., Wider, G., Bushweller, J.H. and Wüthrich, K. (1993) J. Biomol. NMR, 3, 127–132.
Szyperski, T., Pellecchia, M., Wall, D., Georgopoulos, C. and Wüthrich, K. (1994) Proc. Natl. Acad. Sci. USA, 91, 11343–11347.
Vuister, G.W. and Bax, A. (1992) J. Magn. Reson., 98, 428–435.
Vuister, G.W., Kim, S.J., Wu, C. and Bax, A. (1994) J. Am. Chem. Soc., 116, 9206–9210.
Wider, G. and Wüthrich, K. (1993) J. Magn. Reson. Ser. B, 102, 239–241.
Wüthrich, K. (1976) NMR in Biological Research: Peptides and Proteins, North Holland, Amsterdam.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, Wiley, New York, NY.
Yamazaki, T., Kay, J.D. and Kay, L.E. (1993) J. Am. Chem. Soc., 115, 11054–11055.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zerbe, O., Szyperski, T., Ottiger, M. et al. Three-dimensional 1H-TOCSY-relayed ct-[13C,1H]-HMQC for aromatic spin system identification in uniformly 13C-labeled proteins. J Biomol NMR 7, 99–106 (1996). https://doi.org/10.1007/BF00203820
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00203820