Summary
Activity of efferent fibers was recorded from the ramus ophthalmicus superficialis of the head lateral line nerve and the ramus medialis of the trunk lateral line nerve of the axolotl Ambystoma mexicanum. Baseline activity and activity evoked by sensory stimuli were examined. Electrical stimulation of selected branches was used to determine the conduction velocity and the branching pattern of efferent fibers. The influence of lesions at different levels in the CNS on efferent activity was studied.
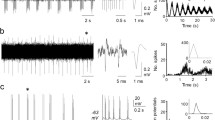
Up to 5 units with baseline activity were found in a single ramus of the lateral line nerve. Discharge rates were variable and highly irregular; they differed between units of the same branch. Bursting activity occurred in 62% of the units. Movements of the animal were accompanied by activity in up to 8 efferent units in a single nerve.
Efferent activity could be elicited or modified by stimulation of visual, labyrinthine, somatosensory, and lateral line systems. Stimulation of the electrosensory system had no effect. Individual efferent neurons innervated different fields in the lateral line periphery. Conduction velocities of efferent fibers ranged from 5 to 12 m/s.
Efferent units received input from various sources at different brain levels up to the diencephalon. These in puts determined the baseline activity. The mechanosensory input was mediated at the medullary level.
Similar content being viewed by others

Abbreviations
- r.m. :
-
ramus medialis
- r.o.s. :
-
ramus ophthalmicus superficialis
- r.s. :
-
ramus superior
References
Art JJ, Kroese ABA (1982) Effects of efferent activity during respiration on Xenopus laevis lateral-line afferent responses. J Physiol 352:21–22
Claas B, Fritzsch B, Münz H (1981) Common efferents to lateral line and labyrinthine systems in aquatic vertebrates. Neurosci Lett 27:231–235
Ellaway PH, Gardner-Medwin AR, Pascoe JE (1983) Decision limits for significance of changes in the cumulative sum (cusum) of the peristimulus time histogram. J Physiol 341:4P-5P
Flock Å, Russell IJ (1976) Inhibition by efferent nerve fibres: Action on hair cells and afferent transmission in the lateral line canal organ of the burbot, Lota lota. J Physiol 257:45–62
Fritzsch B (1981) The pattern of lateral-line afferents in urodeles. Cell Tissue Res 218:581–594
Fritzsch B, Wahnschaffe U (1987) Electron microscopical evidence for common inner ear and lateral line efferents in urodeles. Neurosci Lett 81:48–52
Fukuda J (1974) Fiber composition of the posterior lateral-line nerve of goldfish, investigated by electrophysiological and microscopical techniques. J Comp Neurol 155:203–217
Harris GG, Bergeijk WA van (1962) Evidence that the lateral-line organ responds to near-field displacements of sound sources in water. J Acoust Soc Am 34:1813–1841
Hartmann R, Klinke R (1980) Efferent activity in goldfish vestibular nerve and its influence on afferent activity. Pflügers Arch 388:123–128
Highstein SM, Baker R (1985) Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J Neurophysiol 243:309–325
Lannoo MJ (1987) Neuromast topography in urodele amphibians. J Morphol 191:247–263
Münz H, Claas B, Fritzsch B (1984) Electroreceptive and mechanoreceptive units in the lateral line of the axolotl Ambystoma mexicanum. J Comp Physiol A 154:33–44
Roberts BL, Russell IJ (1972) The activity of lateral-line neurons in stationary and swimming dogfish. J Exp Biol 57:435–448
Roberts BL, Meredith GE (1989) The efferent system. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line. Springer, Berlin Heidelberg New York, pp 445–459
Rudolph P (1967) Zum Ortungsverhalten von Gyrinus substraticus Steph. (Taumelkäfer). Z Vergl Physiol 50:341–361
Russell IJ (1971) The role of lateral-line efferent system in Xenopus laevis. J Exp Biol 54:621–641
Russell IJ, Roberts BL (1972) Inhibition of spontaneous lateral-line activity by efferent nerve stimulation. J Exp Biol 57:77–82
Russell I, Lowe DA (1983) The effect of efferent stimulation on the phase and amplitude of extracellular receptor potentials in the lateral line of the perch (Perca fluviatilis). J Exp Biol 102:223–238
Schmidt RS (1965) Amphibian acoustico-lateralis efferents. J Cell Comp Physiol 65:155–162
Will U, Fritzsch B (1988) The eighth nerve of amphibians: peripheral and central distribution. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 159–184
Zittlau KE, Claas B, Münz H (1986) Directional sensitivity of lateral line units in the clawed toad Xenopus laevis Daudin. J Comp Physiol A 158:469–477
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Münz, H., Claas, B. Activity of lateral line efferents in the axolotl (Ambystoma mexicanum). J Comp Physiol A 169, 461–469 (1991). https://doi.org/10.1007/BF00197658
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00197658