Abstract
The overall therapeutic equivalence of a fluorochlorohydrocarbon (FCH)-free glyceryl trinitrate (GTN) pump spray with a low ethanol content (TL) was investigated relative to an FCH-containing GTN spray (Nitrolingual; R), in terms of: (1) pharmacokinetic bioavailability, (2) pharmacodynamic responses as assessed by digital plethysmography (DPG), and (3) clinical perception upon application.
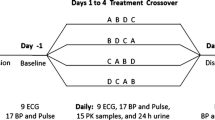
Pharmacokinetically, the time courses of the plasma concentrations of GTN and its dinitrate metabolites, 1,2- and 1,3-GDN, subsequent to the sublingual administration of 0.8 mg GTN showed somewhat lower bioavailability of GTN and its metabolites than to the reference. Pharmacodynamically, the changes in the DPG signals after the application of 0.8 mg GTN with TL were biostatistically euivalent with R (estimated ratio TL/R for the maximum decrease of the ratio between the systolic a wave and c incisure: 0.98; 90% CI: 0.84–1.14; and for the average decrease of the c: a ratio: 0.97; 90% CI: 0.80–1.16). The time of occurrence of the maximum effect of TL was not significantly different from that of R (estimated difference TL-R: -2.25 min; 95% CI:-9.5 min to 2 min).
In contrast, after the administration of an FCH-free GTN spray with a higher ethanol content (TH, active control), the effect had a slightly earlier onset (TH-R:-6 min, 95% CI: -9.5 to -2 min) and there was a higher average response (TH/R: 1.12: 90% CI: 0.95 to 1.34). However, TH was consistently judged to cause an extremely unpleasant burning sensation in the mouth and thus was perceived as distinctly different from R. In contrast, TL was well tolerated and could not be distinguished from R.
Therefore only TL met the criteria of overall therapeutic equivalence to R.
Similar content being viewed by others
References
Murell W (1879) Nitroglycerin as a remedy for angina pectoris. Lancet I:80, 113, 225, 284, 642–646
Thadani U, Opie LH. Nitrates (1987) In: Opie LH, Chatterjee K, Gersh BJ, Harrison DC, Kaplan NM, Marcus FI, Singh BN, Sonnenblick EH, Thadani U (eds) Drugs for the heart. Grune & Stratton, Orlando, pp 19–33
Silber S (1990) Nitrates: why and how should they be used today?. Eur J Clin Pharmacol 38[Suppl 1]:S35-S51
Nast HP (1988) Hypertensive Krise: Nitroglycerin- oder Nifedipin-Kapseln geben? Therapiewoche 38:3898–3903
Scholze J, Matzke J, Linss G (1991) Nitroglyzerin in der Behandlung hypertensiver Krisen bei niereninsuffizienten Patienten. Nieren Hochdruckkrankh 20:701–707
Mügge A, Förstermann U, Lichtlen PR (1989) Endotheliale Funktionen bei kardiovaskulären Erkrankungen. Z Kardiol 78:147–160
Lüscher TF (1989) Endothelium-derived relaxing and contracting factors: potential role in coronary artery disease. Eur Heart J 10:847–857
Sanders PW (1992) Role of nitric oxide in regulation of blood pressure. J Nephrol 5:23–30
Abrams J, Abuquerque NM (1985) Hemodynamic effects of nitroglycerin and long-acting nitrates. Am Heart J 110:216–224
Winsor T, Karpman HL (1958) Waves of the digital plethysmogram. Angiology 9:202–207
Gumbleton M; Benet LZ (1991) Pharmacological activity of the dinitrate metabolites of nitroglycerin following their oral administration to healthy volunteers. Br J Clin Pharmacol 31:211–213
Sickmüller B (1990) FCKW: weitere nationale und internationale Einschränkungen vorgesehen. Pharm Ind 52:290–308
FCKW-Aerosole im medizinischen Bereich (1989) Gegenwärtiger Diskussionsstand und Positionspapier des Bundesverbandes der Pharmazeutischen Industrie e. V. Pharm Ind 51:616–632
Blume H et al (1991) ZL-Monographie Glceroltrinitrat (GTN). Dtsch Apoth Ztg 131:367
Huber T, Merz PG, Harder S, Rietbrock N (1990) Bioequivalence of sublingual glycerol trinitrate. Bioavailability and haemodynamic effects after application of a fluorochlorohydrocarbons-dependent spray and a new pumping system. Drug Res 40:1319–1322
Huber T, Merz MG, Harder S (1991) Bioverfügbarkeit und hämodynamische Wirkungen nach Applikation von sublingualem Glyceroltrinitrat. Ein neues Freisetzungssystem. Drug Res 41:715–718
Erb K, de Mey C, Zimmermann T, Mutschler H, Roll S, Belz GG (1994) Pharmakodynamische Äquivalenz und klinische Verträglichkeit eines neuen FCKW-freien Glyceroltrinitratsprays mit niedrigem Ethanol-Gehalt im Vergleich zu einem FCKW-haltigen Referenzspray und einem FCKW-freien Pumpspray mit höherem Ethanol-Gehalt. Arzneim Forsch 44:478–482
Good clinical practice for trials on medicinal products in the European Community. Note for Guidance II/3976/88-EN, 4.5.90
Lund F (1986) Digital pulse plethysmography (DPG) in studies of the hemodynamic response to nitrates — a survey of recording methods and principles of analysis. Acta Pharmacol Toxicol 59(suppl. VI):79–96
V. Kries J (1892) Studien zur Pulslehre. Mohr, Freiburg, pp 119–126)
Erb KA, de Mey C, Wolf GK, Kroll M, Belz GG (1991) Prüfung der pharmakodynamischen Äquivalenz Glyceroltrinitrathaltigen Sprays zur oralen Anwendung mit und ohne Fluor-Chlor-Kohlenwasserstoffe. Arzneimittelforschung/Drug Res 41:484–488
Porchet H, Bircher J (1982) Noninvasive assessment of portal-systemic shunting: evaluation of a method to investigate systemic availability of oral nitroglyceryl trinitrate by digital plethysmography. Gastroenterology 82:629–637
Isenschmid M, Müller M, Bührer, Vorkauf H, Bircher J (1985) Absolute bioavailability of glyceryl trinitrate from a transdermal system, assessed by digital plethysmography. Int J Clin Pharmacol Ther Tox 23:345–351
Belz GG (1985) Nitrattherapie. In: Hochrein H, Bussmann WD (eds) Perimed, Erlangen, pp 55–61
Yee KF (1986) The calculation of probabilities in rejecting bioequivalence. Biometrics 42:961–965
Meineke I (1987) A simple BASIC program for the calculation of nonparametric confidence intervals in bioequivalence testing. Comp Methods Progr Biomed 24:65–71
Meineke I, de Mey C (1991) Another simple BASIC-program for the assessment of bioequivalence in a two-period crossover design. Comp Methods Progr Biomed 35:1521-RR
APV Guideline (1987) Studies on bioavailability and bioequivalence. Drugs Made in Germany 30:161–166
CPMP Working Party on the Efficacy of Medicinal Products (1991) Note for guidance: investigation of bioavailability and bioequivalence. Commission of the European Communities, III/5489-EN
Food and Drug Administration (1977) Drug products: bioequivalence requirements and in-vivo bioavailability (Dept., Health, FDA). Fed Regi III, 42:1624–1653
Food and Drug Administration (1984) Code of federal regulations. General provisions: procedures for determining the bioavailability of drug products: bioequivalence requirements. FDA, Washington DC 21:117–135
Food and Drug Administration (1988) Report by the bioequivalence task force on recommendations from the bioequivalence hearing conducted by the FDA (29 Sep.-1 Oct 1986) FDA, Washington DC
Nordic Council on Medicines (1987) Bioavailability studies in man. Nordic guidelines. NLN Publication 18, Nordiska Läkemedelsnämnden, Upsala
Schulz HU, Steinijans VW (1991) Striving for standards in bioequivalence assessment: a review. Int J Clin Pharmacol Ther Toxicol 29:293–298
Jähnchen E (1986) Relationship between pharmacokinetics and pharmacodynamics of organic nitrates. Z Kardiol 75[Suppl 3]:12–15
Bogaert M (1987) Clinical pharmacokinetics of glyceryl trinitrate following the use of systemic and topical preparations. Clin Pharmacokinet 12:1–11
Jaeger H et al. (1987) Determination of nitrates in plasma. Drugs 33:9–22
Food and Drug Administration (1977) Single-entity coronary vasodilators. Drugs for human use; drug efficacy study implementation. Permission for drugs to remain on the market. Amendment. Fed Reg 42:43127–43131
Smolen VF (1976) Theoretical and computational basis for drug bioavailability determinations using pharmacological data. I. General considerations and procedures. J Pharmacokinet Biopharm 1976;4:337–353
Smolen VF (1976) Theoretical and computational basis for drug bioavailability determinations using pharmacological data. II. Drug input-response relationships. J Pharmacokinet Biopharm 4:355–375
Smolen VF (1978) Bioavailability and pharmacokinetic analysis of drug responding systems. Ann Rev Pharmacol Toxicol 18:495–522
Buschmann M, Wiegand A, Schnellbacher K, Bonn R, Rehe A, Trenk D, Jähnchen E, Roskamm H (1993) Comparison of the effects of two different galenical preparations of glyceryl trinitrate on pulmonary artery pressure and on the finger pulse curve. Eur J Clin Pharmacol 44:451–456
Smolen VF (1978) Bioavailability and pharmacokinetic analysis of drug responding systems. Ann rev Pharmacol Toxicol 18:495–522
Dollery CT (1973) Editorial. Eur J Clin Pharmacol 6:1
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Mey, C., Erb, K., Mutschler, H. et al. Clinical pharmacological equivalence of a novel FCH-free GTN spray with low ethanol content vs a FCH-containing GTN spray. Eur J Clin Pharmacol 47, 437–443 (1995). https://doi.org/10.1007/BF00196858
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196858