Summary
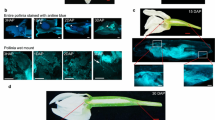
The sperm cells of Rhododendron laetum and R. macgregoriae differentiate within the pollen tube about 24 h after germination in vitro. Threedimensional reconstruction shows that the sperm cells are paired together, and both have extensions that link with the tube nucleus, forming a male germ unit. Quantitative analysis shows that the sperm cells in each pair differ significantly in surface area, but not in cell volume nor in numbers of mitochondria or plastids. When isolated from pollen tubes by osmotic shock, the sperm cells became ellipsoidal and surrounded by their own plasma membrane, while a proportion remained in pairs linked by the inner tube plasma membrane. Both generative and sperm cells are visualized in pollen tube preparations by immunofluorescence with anti-tubulin and anti-actin monoclonal antibodies (MAbs) combined with H33258 fluorescence of the nuclei. Video-image processing shows the presence of an axial microtubule cage in the generative cells, and some microtubules are present in the cytoplasmic extensions that clasp the tube nucleus. Following sperm cell division, the extensive phragmoplast between the sperm nuclei is partitioned by the plasma membranes.
Similar content being viewed by others
References
Brewbaker JL, Kwack BY (1963) The essential role of calcium in pollen germination and tube growth. Am J Bot 50:859–865
Coleman AW, Maguire M, Coleman JR (1981) Mithramycin and 4′,6′-diamidino-2-phenylindole (DAPI) staining for fluorescence microspectrophotometric measurement of DNA in nuclei, plastids and virus particles. J Histochem Cytochem 29:959–968
Cresti M, Ciampolini F, Kapil RN (1984) Generative cells of some angiosperms with particular emphasis on their microtubules. J Submicrosc Cytol 16:317–326
Derksen J, Pierson ES, Traas JA (1985) Microtubules in vegetative and generative cells of pollen tubes. Eur J Cell Biol 38:112–118
Dumas C, Knox RB, McConchie CA, Russell SD (1984) Emerging physiological concepts in fertilization. What's New In Plant Physiol 15:17–20
Franke W, Herth W, Woude W van der, Morre D (1972) Tubular and filamentous structures in pollen tubes: possible involvement as guide elements in protoplasmic streaming and vectorial migration of secretory vesicles. Planta 105:317–341
Heslop-Harrison J (1988) The pollen tube: motility and cytoskeleton. In: Cresti M, Gori P, Pacini E (eds) Sexual reproduction in higher plants. Springer, Berlin Heidelberg New York pp 195–203
Heslop-Harrison J, Heslop-Harrison Y, Cresti M, Tiezzi A, Moscatelli A (1988) Cytoskeletal elements, cell shaping and movement in the angiosperm pollen tube. J Cell Sci 91:44–60
Hough T, Singh MB, Smart IJ, Knox RB (1986) Immunofluorescent screening of monoclonal antibodies to surface antigens of animal and plant cells bound to polycarbonate membranes. J Immunol Methods 92:103–107
Hu S, Zhu C, Xu L (1981) Ultrastructure of male gametophyte in wheat. II. Formation and development of sperm cell. Acta Bot Sin 23:87–91
Kaul V, Theunis CH, Palser BF, Knox RB, Williams EG (1987) Association of the generative cell and vegetative nucleus in pollen tubes of Rhododendron. Ann Bot (London) 59:227–235
Knox RB, Southworth D, Singh MB (1988) Sperm cell determinants and control of fertilisation in plants. In: Chapman GP, Ainsworth CC, Chatham CJ (eds) Eukaryote cell recognition. Concepts and model systems. Cambridge University Press, Cambridge, pp 175–193
Lin J, Uwate WJ, Stallman V (1977) Ultrastructural localization of acid phosphatase in pollen tube of Primus avium L. (sweet cherry). Planta 135:183–190
Maheshwari P (1950) An introduction to the embryology of angiosperms. McGraw-Hill, New York
Marc J, Gunning BES (1986) Immunofluorescent localization of cytoskeletal tubulin and actin during spermatogenesis in Pteridium aquilinum (L.) Kuhn. Protoplasma 134:163–177
Marc J, Gunning BES (1988) Monoclonal antibodies to a fern spermatozoid detect novel components of the mitotic and cytokinetic apparatus in higher plant cells. Protoplasma 142:15–24
Marc J, Gunning BES, Hardham AR, Perkin JL, Wick SM (1988) Monoclonal antibodies to surface and cytoskeletal components of the spermatozoid of Pteridium aquilinum. Protoplasma 142:5–14
McConchie CA, Jobson S, Knox RB (1985) Computer-assisted reconstruction of the male germ unit in pollen of Brassica campes tris. Protoplasma 127:57–63
McConchie CA, Russell SD, Dumas C, Tuohy M, Knox RB (1987) Quantitative cytology of the sperm cells of Brassica campestris and B. oleracea. Planta 170:446–452
Mogensen HL, Rusche ML (1985) Quantitative ultrastructural analysis of barley sperm. I. Occurrence and mechanism of cytoplasm and organelle reduction and the question of sperm dimorphism. Protoplasma 128:1–13
Palevitz BA, Cresti M (1988) Microtubule organization in the sperm of Tradescantia virginiana. Protoplasma 146:28–34
Pierson ES, Derksen J, Traas JA (1986) Organization of microfilaments and microtubules in pollen tubes grown in vitro or in vivo in various angiosperms. Eur J Cell Biol 41:14–18
Raudaskoski M, Astrom H, Perttila K, Virtanen I, Louhelainen J (1987) Role of the microtubule cytoskeleton in pollen tubes: an immunocytochemical and ultrastructural approach. Biol Cell 61:177–188
Russell SD (1984) Ultrastructure of the sperm of Plumbago zeylanica. II. Quantitative cytology and three-dimensional organization. Planta 162:385–391
Russell SD (1985) Preferential fertilization on Plumbago: ultrastructural evidence for gamete level recognition in an angiosperm. Proc Natl Acad Sci USA 82:6129–6132
Russell SD, Cass DD (1983) Unequal distribution of plastids and mitochondria during sperm cell formation in Plumbago zeylanica. In: Mulcahy DL, Ottaviano E (eds) Pollen. Biology and implications for plant breeding. Elsevier, Amsterdam New York, pp 135–140
Shivanna KR, Xu H, Taylor P, Knox RB (1988) Isolation of sperms from the pollen tubes of flowering plants during fertilization. Plant Physiol 87:647–650
Spurr A (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Staff IA, Taylor P, Kenrick J, Knox RB (1989) Ultrastructural analysis of plastids in angiosperm pollen tubes. Sex Plant Reprod 2:70–76
Theunis CA, McConchie CA, Knox RB (1985) Three-dimensional reconstruction of the generative cell and its wall connection in mature bicellular pollen of Rhododendron. Micron Microsc Acta 16:225–231
Tiezzi A, Cresti M, Ciampolini F (1986) Microtubules in Nicotiana pollen tubes: ultrastructural, immunofluorescence and biochemical data. In: Cresti M, Dallai R (eds) Biology of reproduction and cell motility in plants and animals. University of Siena, Siena pp 87–94
Tiezzi A, Moscatelli A, Ciampolini F, Milanesi C, Murgia M, Cresti M (1988) The cytoskeletal apparatus of the generative cell in several angiosperm species. In: Cresti M, Gori P, Pacini E (eds) Sexual reproduction in higher plants. Springer, Berlin Heidelberg New York, pp 215–220
Tilney-Bassett RAE (1978) The inheritance and genetic behaviour of plastids. In: Kirk J, Kirk D, Tilney-Bassett RAE (eds) The plastid. Elsevier/North Holland, Amsterdam New York, pp 251–524
Williams EG, Ramm-Anderson S, Dumas C, Mau S, Clarke AE (1982) The effect of isolated components of Prunus avium L. styles on in vitro growth of pollen tubes. Planta 156:517–519
Wilms HJ (1986) Dimorphic sperm cells in the pollen grain of Spinacia. In: Cresti M, Dallai R (eds) Biology of reproduction and motility in plants and animals, University of Siena, Siena, pp 193–198
Wilms HJ, Van Aelst AC (1983) Ultrastructure of spinach sperm cells in mature pollen. In: Erdelska O (ed) Fertilization and embryogenesis in ovulated plants, Czech Centre Biol Ecol Sci, Bratislava, pp 105–112
Wilms HJ, Leferink-ten Klooster HB (1983) Ultrastructural changes of spinach sperm cells during the progamic phase. Czech Centre Biol Ecol Sci, Bratislava, pp 239–240
Zhu C, Hu S, Xu L, Li X, Shen J (1980) Ultrastructure of sperm cell in mature pollen grain of wheat. Sci Sin 23:371–376
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taylor, P., Kenrick, J., Li, Y. et al. The male germ unit of Rhododendron: quantitative cytology, three-dimensional reconstruction, isolation and detection using fluorescent probes. Sexual Plant Reprod 2, 254–264 (1989). https://doi.org/10.1007/BF00195585
Issue Date:
DOI: https://doi.org/10.1007/BF00195585