Summary
The effect of thermal stimulation on primary afferent neurons and its modulation by Ruthenium Red (RR) has been investigated in the isolated perfused rabbit ear with intact neuronal connection to the animal.
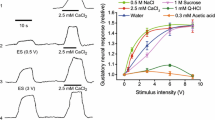
Capsaicin, K+-depolarization as well as increasing the temperature of the perfusate to 50°C, increased the amount of substance P-like immunoreactivity (SP-IR) in the outflow in a calcium-dependent manner. High performance liquid chromatography (HPLC) revealed that SP-IR which was released by thermal stimulation consisted of two components, one of which co-eluted with synthetic substance P. The same two components of SP-IR were also present in extracts of the auricular nerve and were released by capsaicin.
RR attenuated the effect of capsaicin and thermal stimulation but did not reduce potassium-evoked release of SPIR.
To evaluate an inhibitory action of RR on the excitation of primary afferents, the isolated perfused ear with intact neuronal connection to the anaesthetized rabbit was used. Intraarterial injection of capsaicin or bradykinin as well as superfusion of a skin area of approximately 2 cm2 with water at 53°C for 1 min, produced a depressor reflex. RR attenuated the response to thermal stimulation and to capsaicin, but did not block the bradykinin-induced depressor reflex.
These results demonstrate that, in the rabbit ear, thermal stimuli excite primary afferent neurons and evoke the calcium-dependent release of neuropeptides from their peripheral terminals by a mechanism which is sensitive to RR.
Similar content being viewed by others
References
Adams DJ, Takeda K, Umbach JA (1985) Inhibitors of calcium buffering depress evoked transmitter release at the squid giant synapse. J Physiol (Lond) 369:145–149
Alnaes E, Rahamimoff R (1975) On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol (Lond) 248:285 -306
Amann R, Lembeck F (1989) Ruthernium Red selectively prevents capsaicin-induced nociceptor stimulation. Eur J Pharmacol 161:227–229
Amann, R, Skofitsch G, Lembeck F (1988) Species-related differences in the capsaicin-sensitive innervation of the rat and guinea-pig ureter. Naunyn-Schmiedeberg's Arch Pharmacol 338:407–410
Amann R, Donnerer J, Lembeck F (1989a) Ruthenium red selectively inhibits capsaicin-induced release of calcitonin gene-related peptide from the isolated perfused guinea-pig lung. Neurosci Lett 101:311–315
Amann R, Donnerer J, Lembeck F (1989b) Capsaicin-induced stimulation of polymodal nociceptors is antagonized by Ruthenium Red independently of extracellular calcium. Neuroscience 32:255–259
Bernath S, Vizi ES (1987) Inhibitory effect of ionized free calcium enhanced by ruthenium red and m-chloro-carbonylcyanide phenyl hydrazon on the evoked release of acetylcholine. Biochem Pharmacol 38:179–226
Dray A, Bettaney J, Forster P (1989) Capsaicin desensitization of peripheral nociceptive fibres does not impair sensitivity to other noxious stimuli. Neurosci Lett 99:50–54
Gamse R, Molnar A, Lembeek F (1979) Substance P release from spinal cord by capsaicin. Life Sci 25:629–636
Geppetti P, Maggi CA, Perretti F, Frilli S, Manzini S (1988) Simultaneous release by bradykinin of substance P- and calcitonin gene-releated peptide immunoreactivities from capsaicin-sensitive structures in guinea-pig heart. Br J Pharmacol 94:288–290
Gulbekian S, Merighi A, Wharton J, Varndell IM, Polak JM (1986) Ultrastructural evidence for the coexistence of calcitonin gene-related peptide and substance P in secretory vesicles of peripheral nerves in the guinea-pig. J Neurocytol 15:535–542
Helme RD, Koschorke GM, Zimmermann M (1986) Immunoreactive substance P release from skin nerves in the rat by noxious thermal stimulation. Neurosci Lett 63:295–299
Heyman I, Rang HP (1985) Depolarizing responses to capsaicin in a subpopulation of dorsal root ganglion cells. Neurosci Lett 56:69–75
Holzer P (1988) Local effector function of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24: 739–768
Holzer P, Lippe ITH (1988) Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience 27:981–987
Hua X-Y, Saria A, Gamse R, Theodorsson-Norheim E, Brodin E, Lundberg JM (1986) Capsaicin induced release of multiple tachykinins (substance P, neurokinin A and cledoisin-like material) from guinea-pig spinal cord and ureter. Neuroscience 19:313–319
James IF, Walpole CSJ, Coote PR, Wood JN (1989) Immunechemical detection of photoaffinity-labelled capsaicin binding proteins from sensory neurons (abstract). International Workshop on Sensory Neuropeptides, Florence, June 1989
Juan H (1977) Inhibition of the algesic effect of bradykinin and acetylcholine by mepacrine: Naunyn-Schmiedeberg's Arch Pharmacol 301: 23–27
Juan H, Lembeck F (1974) Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn-Schmiedeberg's Arch Pharmacol 283:151–164
Juan H, Lembeck F, Seewann S, Hack U (1980) Nociceptor stimulation and PGE release by capsaicin. Naunyn-Schmiedeberg's Arch Pharmacol 312:139–143
Lembeck F, Bernatzky G, Gamse R, Saria A (1985) Characterization of substance P-like immunoreactivity in submammalian species by high performance liquid chromatography. Peptides 6 [Suppl 3]:231 - 236
Madeira VMC, Autunes-Madeira MC (1973) Interaction of Ca2+ and Mg2+ with synaptic plasma membranes. Biochim Biophys Acta 232:396–407
Maggi CA, Meli A (1988) The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol 19:1–43
Maggi CA, Evangelista S, Giuliani S, Meli A (1987) Anti-ulcer activity of calcitonin gene-related peptide in rats. Gen Pharmacol 18:33–34
Maggi CA, Santicioli P, Geppetti P, Parlani M, Astolfi M, Pradeles P, Patacchini R, Meli A (1988) The antagonism induced by Ruthenium Red of the actions of capsaicin on the peripheral terminals of sensory neurons: further studies. Eur J Pharmacol 154:1–10
Maggi CA, Giuliani S, Meli A (1989a) Effect of Ruthenium Red on responses mediated by activation of capsaicin-sensitive nerves of the rat urinary bladder. Naunyn-Schmiedeberg's Arch Pharmacol 340:541–546
Maggi CA, Patacchini R, Santicioli P, Giuliani S, DelBianco E, Geppetti P, Meli A (1989b) The “efferent” function of capsaicin-sensitive nerves: Ruthenium Red discriminates between different modes of activation. Eur J Pharmacol 170:167–177
Maggi CA, Santicioli P, Geppetti P, Parlani M, Astolfi M, DelBianco E, Patacchini R, Giuliani S, Meli A (1989c) The effect of calcium free medium and nifedipine on the release of substance P-like immunoreactivity and contractions induced by capsaicin in the isolated guinea-pig bladder. Gen Pharmacol 20:445–456
Marsh SJ, Stansfield CE, Brown DA, Davey R, McCarthy D (1987) The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience 23:275–289
Moore CL (1971) Specific inhibition of mitochondrial Ca 2+ transport by ruthenium red. Biochem Biophys Res Commun 42: 298–305
Reed KC, Bygrave FL (1974) The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J 140:143–155
Saria A (1984) Substance P in sensory nerve fibres contributes to the development of oedema in the rat hind paw after thermal injury. Br J Pharmacol 82:217–222
Saria A, Gamse R, Petermann J, Fischer JA, Theodorsson-Norheim E, Lundberg JM (1986) Simultaneous release of several tachykinins and calcitonin gene-related peptide from rat spinal cord slices. Neurosci Lett 63:310–314
Saria A, Martling C-R, Yan Z, Theodorsson-Norheim E, Gamse R, Lundberg JM (1988) Release of multiple tachykinins from capsaicin-sensitive sensory nerves in the lung by bradykinin, histamine, dimethylphenyl piperazinium, and vagal nerve stimulation. Am Rev Respir Dis 137:1330–1335
Schweitzer A, Brom R (1985) Differentiation of peripheral and central effects of algesic drugs. Int J Tissue React 7:79–83
Swanson PD, Anderson L, Stahl WL (1974) Uptake of calcium ions by synaptosomes from rat brain. Biochim Biophys Acta 356:174–183
Szolcsanyi J (1984) Capsaicin-sensitive chemoceptive neural system with dual sensory-efferent function. In: Chahl LA, Szolcsanyi J, Lembeck F (eds) Antidromic vasodilatation and neurogenic inflammation. Akademia Kiado, Budapest, pp 27–52
Szolcsanyi J (1987) Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultraviolett irradiation. J Physiol (Lond) 388:9–23
Szolcsanyi J, Bartho L (1981) Impaired defense mechanism to peptic ulcer in the capsaicin-desensitized rat. In: Mozsik G, Hänninen O, Javor T (eds) Gastrointestinal defense mechanisms. Pergamon, Oxford, Akademia Kiado, Budapest, pp 39–51
Szolcsanyi J, Jancso-Gabor A (1975) Sensory effects of capsaicin congeners. Drug Res 25:1877–1881
Tapia R, Meza-Ruiz G (1977) Inhibition by Ruthenium Red of the calcium-dependent release of 3H-GABA in synaptosomal fractions. Brain Res 126:160–166
Toresson G, Brodin E, Wahlström A, Bertilsson L (1988) Detection of N-terminally extended substance P but not of substance P in human cerebrospinal fluid: quantitation with HPLC-radioimmunoassay. J Neurochem 50:1701–1707
Tsunoo A, Konishi S, Otsuka M (1982) Substance P as an excitatory transmitter of primary afferent neurons in guinea-pig sympathetic ganglia. Neuroscience 7:2025–2037
Wood JN, Winter J, James JF, Rang HP, Yeats J, Bevan S (1988) Capsaicin-induced ion fluxes in dorsal root ganglionic cells in culture. J Neurosci 8:3208–3220
Author information
Authors and Affiliations
Additional information
Send offprint requests to R. Amann at the above address
Rights and permissions
About this article
Cite this article
Amann, R., Donnerer, J. & Lembeck, F. Activation of primary afferent neurons by thermal stimulation. Naunyn-Schmiedeberg's Arch Pharmacol 341, 108–113 (1990). https://doi.org/10.1007/BF00195066
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195066