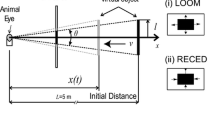
Summary
The results of previous behavioral studies can be so interpreted that the prey-catching behavior in the toad is elicited if there is a ‘local’ motion restricted with-in a small part of the visual field, while it is suppressed if there is a ‘global’ motion over a large part of the visual field. This has led us to design experiments to answer a specific question (yet a very essential one for understanding neural processes underlying this behavior): Are there ‘local motion detectors’ in the toad's visual system that are not activated by ‘global’ motion over a large part of the visual field but are activated by ‘local’ motion confined within a smaller part of it? The present study showed that (1) the majority of the toad's tectal neurons exhibit properties of the ‘local motion detectors’ as defined above, and (2) these properties can be explained from the receptive field structure revealed in the present experiments. Based on these results, we suggest that the tectal ‘local motion detectors’ are essential for the detection and localization of small moving prey-objects in the natural environment while ignoring the large moving objects or the self-induced motion of the visual field.
Similar content being viewed by others
Abbreviations
- ERF :
-
excitatory receptive field
- G1-5 :
-
group 1–5 neurons
References
Allman J, Miezin F, McGuinness E (1985) Direction- and velocity-specific responses from beyond the classical receptive field in the middle temporal visual area (MT). Perception 14:105–126
Burghagen H, Ewert J-P (1983) Influence of the background for discriminating object motion from self-induced motion in toads Bufo bufo (L.). J Comp Physiol 152:241–249
Cott HB (1957) Adaptive coloration in animals. Methuen, London
Dean P, Redgrave P, Westby GWM (1989) Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci 12:137–147
Edmunds M (1974) Defense in animals. Longman, London
Egelhaaf M, Hausen K, Reichardt W, Wehrhahn C (1988) Visual course control in flies relies on neuronal computation of object and background motion. Trends Neurosci 11:351–358
Ewert J-P (1984) Tectal functions underlying prey-catching and predator avoidance behaviors in toads. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum Press, New York, pp 247–416
Ewert J-P, Hock F (1972) Movement-sensitive neurones in the toad's retina. Exp Brain Res 16:41–59
Ewert J-P, Wietersheim A von (1974) Musterauswertung durch Tectum- und Thalamus/Praetectum-Neurone im visuellen System der Kröte Bufo bufo (L.). J Comp Physiol 92:131–148
Fite KV (1969) Single-unit analysis of binocular neurons in the frog optic tectum. Exp Neurol 24:475–486
Frost BJ (1982) Mechanisms for discriminating object motion from self-induced motion in the pigeon. In: Ingle DJ, Goodale MA, Mansfield RJW (eds) Analysis of visual behavior. MIT Press, Cambridge, pp 177–196
Frost BJ, Nakayama K (1983) Single visual neurons code opposing motion independent of direction. Science 220:744–745
Frost BJ, Scilley PL, Wong SCP (1981) Moving background patterns reveal double opponency of directionally specific pigeon tectal neurons. Exp Brain Res 43:173–185
Frost BJ, Cavanagh P, Morgan B (1988) Deep tectal cells in pigeons respond to kinematograms. J Comp Physiol A 162:639–647
Gaillard F (1990) Visual units in the central nervous system of the frog. Comp Biochem Physiol 96A:357–371
George A, Grüsser-Cornehls U (1975) Responses of frog tectal cells to moving and stationary visual stimuli. Pflügers Arch (Suppl) 359:203
Gibson JJ (1950) The perception of the visual world. Houghton Mifflin, Boston
Grobstein P (1988) Between the retinotectal projection and directed movement: topography of a sensorimotor interface. Brain Behav Evol 31:34–48
Grüsser O-J, Grüsser-Cornehls U (1976) Physiology of the anuran visual system. In: Llinás R, Precht W (eds) Frog neurobiology. Springer, Berlin, pp 297–385
Grüsser O-J, Grüsser-Cornehls U, Bullock TH (1964) Functional organization of receptive fields of movement detecting neurons in the frog's retina. Pflügers Arch 279:88–93
Grüsser-Cornehls U, Langeveld S (1985) Velocity sensitivity and directional selectivity of frog retinal ganglion cells depend on chromaticity of moving stimuli. Brain Behav Evol 27:165–185
Honigmann H (1944) The visual perception of movement by toads. Proc R Soc Lond B 132:291–307
Horridge GA (1987) The evolution of visual processing and the construction of seeing systems. Proc R Soc Lond B 230:279–292
Ingle D (1976) Behavioral correlates of central visual function in anurans. In: Llinás R, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 435–451
Julesz B (1971) Foundations of Cyclopean perception. University of Chicago Press, Chicago
Lettvin JY, Maturana HR, McCulloch WS, Pitts WH (1959) What the frog's eye tells the frog's brain. Proc Inst Radio Engen 47:1940–1951
Lock A, Collett T (1979) A toad's devious approach to its prey: a study of some complex uses of depth vision. J Comp Physiol 131:179–189
Loomis JM, Nakayama K (1973) A velocity analogue of brightness contrast. Perception 2:425–428
Maeda M, Magherini PC, Precht W (1977) Functional organization of vestibular and visual inputs to neck and forelimb motoneurons in the frog. J Neurophysiol 40:225–243
Mandl G (1985) Responses of visual cells in cat superior colliculus to relative pattern movement. Vision Res 25:267–281
Manteuffel G, Fiseifis S (1990) Configuration-sensitive visual responses in the superior colliculus of the house mouse (Mus musculus domesticus). Brain Behav Evol 35:176–184
Matsushima T, Satou M, Ueda K (1989) Medullary reticular neurons in the Japanese toad: morphologies and excitatory inputs from the optic tectum. J Comp Physiol A 166:7–22
Maturana HR, Lettvin JY, McCulloch WS, Pitts WH (1960) Anatomy and physiology of vision in the frog (Rana pipiens). J Gen Physiol 43 (Suppl 2, Mechanisms of Vision):129–171
Metzger W (1953) Gesetze des Sehens. Kramer, Frankfurt am Main
Nakayama K (1985) Biological image motion processing: a review. Vision Res 25:625–660
Neuweiler G (1984) Foraging, echolocation and audition in bats. Naturwissenschaften 71:446–455
Regan D (1986) Form from motion parallax and form from luminance contrast: Vernier discrimination. Spatial Vison 1:305–318
Roth G (1986) Neural mechanisms of prey recognition: an example in amphibians. In: Feder ME, Lauder GV (eds) Predator-prey relationships, perspectives and approaches from the study of lower vertebrates. University of Chicago Press, Chicago, pp 42–68
Roth G, Jordan M (1982) Response characteristics and stratification of tectal neurons in the toad Bufo bufo (L.). Exp Brain Res 45:393–398
Satou M (1991) Local motion processing in the toad's optic tectum. Neurosci Res, Suppl 14:10
Satou M, Ewert J-P (1985) The antidromic activation of tectal neurons by electrical stimuli applied to the caudal medulla oblongata in the toad, Bufo bufo L. J Comp Physiol A 157:739–748
Satou M, Matsushima T, Takeuchi H, Ueda K (1985) Tongue muscle-controlling motoneurons in the Japanese toad: topography, morphology and neuronal pathways from the ‘snapping evoking area’ in the optic tectum. J Comp Physiol A 157:717–737
Satou M, Tsai H-J, Shiraishi A, Ueda K (1988) Influence of textured background upon motion processing in the optic tectum of Japanese toad. Proc Annu Meet Jpn Soc Gen Comp Physiol 10:120
Satou M, Tsai H-J, Shiraishi A, Ueda K (1989) After-effects of moving textured background in motion-sensitive neurons of anuran optic tectum. Brain Res 504:320–324
Sparks DL, Nelson JS (1987) Sensory and motor maps in the mammalian superior colliculus. Trends Neurosci 10:312–317
Stein BE (1984) Multimodal representation in the superior colliculus and optic tectum. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum Press, New York, pp 819–841
Takeuchi H-A, Satou M, Ueda K (1991) Jaw-muscle controlling motoneurons in the Japanese toad: neural inputs from the tectal ‘snapping-evoking area’ and the oral sensory nerves. Brain Res (in press)
Tanaka K, Hikosaka K, Saito H, Yukie M, Fukuda Y, Iwai E (1986) Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci 6:134–144
Tsai H-J (1990) Responses of toad's tectal neurons to in-phase and anti-phase movements of object and textured background. J Comp Physiol A 167:857–863
Tsai H-J, Ewert J-P (1988) Influence of stationary and moving textured backgrounds on the response of visual neurons in toads (Bufo bufo L.). Brain Behav Evol 32:27–38
Tsai H-J, Satou M, Shiraishi A, Ueda K (1989) Effects of moving background on neuronal responses in the toad's optic tectum. Naturwissenschaften 76:37–38
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satou, M., Shiraishi, A. Local motion processing in the optic tectum of the Japanese toad, Bufo japonicus . J Comp Physiol A 169, 569–589 (1991). https://doi.org/10.1007/BF00193547
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00193547