Summary
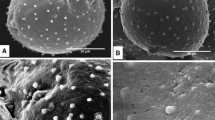
The mature pollen of Larix leptolepis Gord. (Conifer) contains five different cell types, and the plasma membrane of the vegetative cell is continuous and organized. The pollen wall is composed of two morphologically and cytochemically distinct domains: the exine and the intine. In the multilayered exine, the ektexine appears granular and the endexine, lamellar. The intine is thick and bilayered with a microfibrillar structure occupying its inner portion. Cytochemical reactions of the exine and the intine are similar to those found in angiosperms. Pollen wall involvement in the male female recognition system is discussed with respecl to the angiosperms.
Similar content being viewed by others
References
Bronner R (1975) Simultaneous demonstration of lipids and starch in plant tissues. Stain Technol 50:1–4
Chandler C, Mavrodineanu S (1965) Meiosis in Larix laricina Koch. Contrib Boyce Thompson Inst 23:67–76
Christiansen H (1972) On the development of pollen and the fertilization mechanisms of Larix and Pseudotsuga menziesii. Silvae Genet 21:166–174
Dexheimer J (1969) Sur l'étude comparée des ultrastructures des grains de pollen des Angiospermes, des Gymnospermes et des Préphanérogames. Rev Cytol Biol Veg 32:129–140
Dexheimer J (1970) Cytophysiologie du pollen. Rev Cytol Biol Veg 33:169–234
Duhoux E (1972) Evolution structurale de la paroi du grain de pollen du Juniperus communis L. (Cupressacée) cultivé in vitro, au cours de la phase d'hydratation. CR Seances Acad Sci 274:2767–2770
Duhoux E (1980) Le développement cellulaire du tube pollinique du Juniperus communis L. (Cupressacée) cultivé in vitro. Rev Cytol Biol Vég 3:95–145
Dumas C, Knox RB, Gaude T (1984) Pollenpistil recognition: new concepts from electron microscopy and cytochemistry. Int Rev Cytol 90:239–272
Escaig J, Nicolas G (1976) Cryo-fractures de matériel biologique réalisées à très basses températures en ultravide. CR Seances Acad Sci 283:1245–1248
Feder N, O'Brien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142
Fisher DB (1968) Protein staining of ribboned epon sections for light microscopy. Histochemie 16:92–96
Gaude T, Dumas C (1984) A membrane-like-structure on the pollen wall surface in Brassica. Ann Bot (London) 54:821–825
Gullväg BM (1966) The fine structure of some gymnosperm pollen walls. Grana Palynol 6:435–475
Hagman M (1975) Incompatibility in forest trees. Proc R Soc London Ser B 188:313–326
Heslop-Harrison J (1976) The adaptative significance of the exine. In: Ferguson IK, Muller J (eds) The evolutionary significance of the exine. Academic Press, New York London, pp 27–37
Heslop-Harrison J (1979) Aspects of the structure cytochemistry and germination of the pollen of rye (Secale cereale L.) Ann Bot (London), Suppl 1:1–47
Heslop-Harrison J, Heslop-Harrison Y, Knox RB, Howlett B (1973) Pollen-wall proteins. Gametophytic and sporophytic fractions in the pollen walls of the Malvaceae. Ann Bot (London) 37:403–412
Hesse M (1980) Pollenkitt is lacking in Gnetum gnemon (Gnetaceae). Plant Syst Evol 136:41–46
Ho RH, Rouse GE (1970) Pollen germination of Larix sibirica (Siberian larch) in vitro. Can J Bot 48:213–215
Jensen WA (1962) Carbohydrates and cell walls constituents. In: Whitaker DM (ed) Botanical histochemistry. Freeman, San Francisco London, pp 175–208
Kaji K (1974) On the pollination and development of ovules and on the sterility of seeds in Japanese larch (Larix leptolepis Gord). Bull Hokkaido For Exp Stn 12:1–12
Kerhoas C, Gay G, Dumas C (1978) A multidisciplinary approach to the study of the plasma membrane of Zea mays pollen during controlled dehydration. Planta 171:1–10
Knox RB (1979) Pollen and allergy. (Studies in biology, no 107). Arnold, London
Knox RB (1984) The pollen grain. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York, pp 197–271
Knox RB, Heslop-Harrison J (1970) Pollen wall proteins: localization and enzymic activity. J Cell Sci 6:1–27
Kosinski G (1986) Megagametogenesis, fertilization and embryo development in Larix decidua. Can J For Res 16:1301–1309
Meyer NR, Bernard VV (1973) Electron microscopic study of pollen grains formation in Pinus sylvestris L., Juniperus cornmunis L., Larix sibirica Ledeb. Proceeding of the IIIrd International Palynological Conference, Novosibirsk, pp 21–24
Moitra A, Bhatnagar SP (1982) Ultrastructure, cytochemical and histochemical studies on Pollen and male gamete development in Gymnosperms. Gamete Res 5:71–112
Owens JN, Blake MD (1985) Forest tree seed production. Petawawa National Forestry Institute Petawawa (Canadian Forestry Service information report)
Owens JN, Molder M (1979) Sexual reproduction of Larix occidentalis. Can J Bot 57:2673–2690
Owens JN, Simpson S (1986) Pollen from conifers native to British Columbia. Can J For Res 16:955–967
Petricevic S, Vidakovic M, Bilic I, Borzan Z (1977) Immunological identity of pollen-wall proteins in some in compatible pine species. Genetika 9:271–280
Pettitt JM (1985) Pollen tube development and characteristics of the protein emission in conifers. Ann Bot (London) 56:309–326
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208
Rowley JR, Flynn JJ (1979) Migration of lanthanum through the pollen wall. Cytobiologie 3:1–12
Said C (1988) Différenciation et reconnaissance des gamétophytes mâle et femelle chez Larix leptolepis: étude structurale. Thesis, Université Claude Bernard, Lyon
Said C, Zandonella P, Villar M (1985) Pollen ovule interactions in Larix leptolepis. New data and hypothesis. In: Mulcahy DL, Bergamini-Mulcahy G, Ottaviano E (eds) Biotechnology and ecology of pollen. Springer, Berlin Heidelberg New York, pp 507–509
Southworth D (1985) Pollen exine substructure. III. Juniperus communis. Can J Bot 64:983–987
Sterling C (1963) The structure of the male gametophyte in Gymnosperms. Biol Rev 38:167–203
Thiery JP (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc 6:987–1018
Ueno J (1960) Studies on pollen grains of gymnospermae. J Inst Polytech Osaka City Univ Ser D 11:109–136
Van Campo M (1971) Précisions nouvelles sur les structures comparées des pollens de Gymnospermes et d'Angiospermes. CR Seances Acad Sci 272:2071–2074
Van Campo M, Lugardon B (1973) Structure grenue infratectale de l'ectexine des pollens de quelques Gymnospermes et d'Angiospermes. Pollen Spores 15:171–187
Vidakovic M (1977) Overcoming the incompatibility in crossing some pine species. Genetika 9:51–63
Villar M, Knox RB, Dumas C (1984) Effective pollination period and nature of pollen-collecting aparatus in the Gymnosperm, Larix leptolepis. Ann Bot (London) 53:279–284
Yamazaki T, Takeoka M (1962) Electron microscope investigations of the fine details of the pollen grain surface in Japanese Gymnosperms. Grana Palynol 3:3–14
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Said, C. Some characteristics of pollen wall cytochemistry and ultrastructure in Japanese larch (Larix leptolepis Gord.). Sexual Plant Reprod 2, 77–84 (1989). https://doi.org/10.1007/BF00191994
Issue Date:
DOI: https://doi.org/10.1007/BF00191994