Abstract
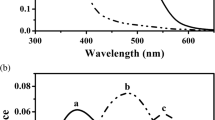
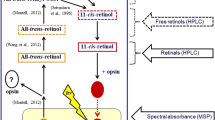
The benthic amphipod Pontoporeia affinis lives in the Baltic sea and in northern European lakes in an environment where very little light is available for vision. The eyes, consisting of 40–50 ommatidia, are correspondingly modified. Microspectrophotometric recordings on isolated eyes show the presence of at least two kinds of screening pigments in the ommatidia with maxima at 540–580 nm and 460–500 nm. Difference spectra obtained from the rhabdoms after exposure to red and blue light, respectively, give evidence of a single rhodopsin with its maximum at 548 nm and a 500-nm metarhodopsin. In ERG recordings sensitivity in the dark-adapted state, after saturating exposures to blue and to red light, stabilizes at levels determined by the rhodopsin concentration. No change is observed during 10–14 h after the beginning of dark adaptation. However, using animals pre-exposed with a strong red light and then kept in darkness, it is found that after a delay of 20–40 h sensitivity of the dark-adapted eye begins to increase and finally, after 5–6 days reaches a level corresponding to 100% rhodopsin. Thus, a slow renewal of rhodopsin appears to occur in darkness, where a photoisomerization of metarhodopsin is excluded.
Similar content being viewed by others
Abbreviations
- ERG:
-
electroretinogram
- IR:
-
infrared
- MSP:
-
microspectrophotometry
References
Almagor E, Hillman P, Minke B (1979) Upper limit on translational diffusion of visual pigment in intact unfixed barnacle photoreceptors. Biophys Struct Mech 5:243–248
Arikawa K, Kawamata K, Suzuki T, Eguchi E (1987) Daily changes of structure, function and rhodopsin content in the compound eye of the crab Hemigrapsus sanguineus. J Comp Physiol A 161:161–174
Barnes SN, Goldsmith TH (1977) Dark adaptation, sensitivity and rhodopsin level in the eye of the lobster, Homarus. J Comp Physiol 120:143–159
Beatty RA (1949) The pigmentation of cavernicolous animals. III. The carotenoid pigments of some amphipod Crustacea. J Exp Biol 26:125–130
Blest AD, Maples J (1979) Exocytotic shedding and glial uptake of photoreceptor membrane by a salticid spider. Proc R Soc Lond 8204:105–112
Bridges CDB (1972) The rhodopsin-porphyropsin visual system. In: Dartnall HJA (ed) Photochemistry of vision (Handbook of sensory physiology, vol VII/1). Springer, Berlin Heidelberg New York, pp 417–480
Briggs MH (1961) Visual pigment of grapsoid crabs. Nature 190:784–786
Bruno MS, Barnes SN, Goldsmith TH (1977) The visual pigment and visual cycle of the lobster, Homarus. J Comp Physiol 120:123–142
Cronin TW, Forward RB Jr (1988) The visual pigments of crabs. I. Spectral characteristics. J Comp Physiol A 162:463–478
Cronin TW, Goldsmith TH (1984) Dark regeneration of rhodopsin in crayfish photoreceptors. J Gen Physiol 84:63–81
Debaisieux P (1944) Les yeux des Crustaces. La Cellule 50:9–122
Denton EJ, Warren FJ (1957) The photosensitive pigments in the retinae of deep-sea fish. J Mar Biol Assoc UK 36:651–662
Donner KO (1971) On vision in Pontoporeia affinis and P. femorata (Crustacea, Amphipoda). Commentat Biol 41:1–17
Donner KO, Lindström M (1980) Sensitivity to light and circadian activity of Pontoporeia affinis (Crustacea, Amphipoda). Ann ZoolFenn 17:203–212
Ebrey TG, Honig B (1977) New wavelength dependent visual pigment nomograms. Vision Res 17:147–151
Eguchi E, Waterman TH (1967) Changes in the retinal fine structure induced in the crab Libinia by light and dark adaptation. Z Zellforsch 79:209–229
Eguchi E, Waterman TH (1976) Freeze-etch and histochemical evidence for cycling in crayfish photoreceptor membranes. Cell Tissue Res 169:419–434
Elofsson R, Hallberg E (1973) Correlation of ultrastructure and chemical composition of crustacean chromatophore pigment. J Ultrastruct Res 44:421–429
Fernandez HR (1973) Spectral sensitivity and visual pigment of the compound eye of the galatheid crab Pleuroncodes planipes. Mar Biol 20:148–153
Forward RB Jr, Cronin TW, Douglass JK (1988) The visual pigments of crabs. II. Environmental adaptations. J Comp Physiol A 162:479–490
Goldsmith TH (1972) The natural history of invertebrate visual pigments. In: Dartnall HJA (ed) Photochemistry of vision (Handbook of sensory physiology, vol VII/1). Springer, Berlin Heidelberg New York, pp 685–719
Goldsmith TH (1978) The effects of screening pigments on the spectral sensitivity of some Crustacea with scotopic (superposition) eyes. Vision Res 18:475–482
Goldsmith TH, Wehner R (1977) Restrictions on rotational and translational diffusion of pigment in the membranes of a rhabdomeric photoreceptor. J Gen Physiol 70:453–490
Green JB (1972) Pigmentation of the eyes of Nebalia bipes Fabr. (Leptostraca). Crustaceana 22:206–207
Hafner GS, Tokarski TR (1988) The diurnal pattern of protein and photopigment synthesis in the retina of the crayfish, Procambarus clarkii. J Comp Physiol A 163:253–258
Hafner GS, Tokarski T, Jones C, Martin R (1982) Rhabdom degradation in white-eyed and wild-type crayfish after long term dark adaptation. J Comp Physiol 148:419–429
Hallberg E, Elofsson R (1989) Construction of the pigment shield of the crustacean compound eye: a review. J Crust Biol 9:359–372
Hallberg E, Nilsson HL, Elofsson R (1980) Classification of amphipod compound eyes — the fine structure of the ommatidial units (Crustacea, Amphipoda). Zoomorphol 94:279–306
Hamdorf K (1979) The physiology of invertebrate visual pigments. In: Autrum H (ed) Invertebrate photoreceptors (Handbook of sensory physiology, vol VII/6). Springer, Berlin Heidelberg New York, pp 145–224
Hamdorf K, Schwemer J (1975) Photoregeneration and the adaptation process in insect photoreceptors. In: Snyder AW, Menzel R (eds) Photoreceptor optics. Springer, Berlin Heidelberg New York, pp 263–289
Hamdorf K, Paulsen R, Schwemer J (1973) Photoregeneration and sensitivity control of photoreceptors of invertebrates. In: Langer H (ed) Biochemistry and physiology of visual pigments. Springer, Berlin Heidelberg New York, pp 155–166
Henning U, Langer H (1986) Untersuchungen zum Turnover der Photorezeptormembran im Auge der Krabbe Ocypode ryderi. Verh Dtsch Zool Ges 79:213
Hiller-Adams P, Widder EA, Case JF (1988) The visual pigments of four deep-sea crustacean species. J Comp Physiol A 163:63–72
Hillman P, Hochstein S, Minke B (1983) Transduction in invertebrate photoreceptors: role of pigment bistability. Physiol Rev 63:668–772
Itaya SJ (1976) Rhabdom changes in the shrimp, Palaemonetes. Cell Tissue Res 166:265–273
Langer H (1975) Properties and functions of screening pigments in insect eyes. In: Snyder AW, Menzel R (eds) Photoreceptor optics. Springer, Berlin Heidelberg New York, pp 429–455
Langer H, Schlecht P, Schwemer J (1982) Microspectrophotometric investigation of insect visual pigments. In: Parker L (ed) Methods in enzymology, vol 81. Academic Press, New York, pp 729–741
Langer H, Pieper M, Henning U (1990) Photoreceptor membranes and visual pigments in the apposition eyes of the terrestrial crab Ocypode ryderi (Ocypodidae) during the daily light cycle. (Proc Internat Crust Conf, Brisbane). Mem Queensland Mus 31:279
Lindström M, Nilsson HL (1983) Spectral and visual sensitivities of Cirolana borealis Lilljeborg, a deep-water isopod (Crustacea: Flabellifera). J Exp Mar Biol Ecol 69:243–256
Lindström M, Nilsson HL (1988) Eye function of Mysis relicta Lovén (Crustacea) from two photic environments. Spectral sensitivity and light tolerance. J Exp Mar Biol Ecol 120:23–37
Lindström M, Nilsson HL, Meyer-Rochow VB (1988) Recovery from light-induced sensitivity loss in the eye of the crustacean Mysis relicta in relation to temperature: a study of ERG-determined V÷log I relationships and morphology at 4 °C and 14 °C. Zool Sci 5:743–757
Lipetz LE, Cronin TW (1988) Application of an invariant spectral form to the visual pigments of crustaceans: implications regarding the binding of the chromophore. Vision Res 28:1083–1093
Loew ER (1976) Light and photoreceptor degeneration in the Norway lobster, Nephrops norvegicus (L.). Proc R Soc Lond B 193:31–44
Makino-Tasaka M, Suzuki T (1986) Quantitative analysis of retinal and 3-dehydroretinal by high-pressure liquid chromatography. Methods Enzymol 123:53–61
Meyer-Rochow VB (1981) The eye of Orchomene sp. cf. O. rossi, an amphipod living under the Ross Ice Shelf (Antarctica). Proc R Soc Lond B 212:93–111
Michel A, Anders F (1954) Über die Pigmente im Auge von Gammarus pulex L. (Crust., Amphipod.). Naturwissenschaften 41:72
Minke B, Kirschfeld K (1978) Microspectrophotometric evidence for two photointerconvertible States of visual pigment in the barnacle lateral eye. J Gen Physiol 71:37–45
Nässei DR, Waterman TH (1979) Massive diurnally modulated membrane turnover in crab light and dark adaptation. J Comp Physiol 131:205–216
Parker GH (1899) The photomechanical changes in the retinal pigment of Gammarus. Bull Mus Comp Zool Harvard Coll 35:143–150
Rosenberg J, Langer H (1993) Feinstruktur der Komplexaugen von Pontoporeia affinis (Amphipoda, Gammaridae) und die Wirkung von Licht auf die Rhabdome. Contrib 6th meeting of German Crustaceologists, Rostock-Warnemünde 1993
Schlecht P, Hamdorf K, Langer H (1978) The arrangement of colour receptors in a fused rhabdom of an insect. J Comp Physiol 123:239–243
Schwemer J (1983) Pathways of visual pigment regeneration in fly photoreceptor cells. Biophys Struct Mech 9:287–298
Schwemer J (1984) Renewal of visual pigment in photoreceptors of the blowfly. J Comp Physiol A 154:535–547
Schwemer J (1986) Turnover of photoreceptor membrane and visual pigment in invertebrates. In: Stieve H (ed) The molecular mechanism of photoreception. Dahlem Konf 1985. Springer, Berlin Heidelberg New York, pp 303–326
Schwemer J (1989) Visual pigments of compound eyes structure, photochemistry, and regeneration. In: Stavenga DG, Hardie RC (eds) Facets of vision. Springer, Berlin Heidelberg New York, pp 112–133
Schwemer J, Langer H (1982) Insect visual pigments. In: Parker L (ed) Methods in enzymology, vol 81. Academic Press, New York, pp 182–197
Segerstråle S (1957) On the immigration of the glacial relicts of Northern Europe, with remarks on their prehistory. Commentat Biol 16:1–117
Smith WC, Goldsmith TH (1991) The role of retinal photoisomerase in the visual cycle of the honeybee. J Gen Physiol 97:143–165
Stavenga DG (1989) Pigments in compound eyes. In: Stavenga DG, Hardie RC (eds) Facets of vision. Springer, Berlin Heidelberg New York, pp 152–172
Stavenga DG, Schwemer J (1984) Visual pigments of invertebrates. In: Ali MA (ed) Photoreception and vision in invertebrates. Plenum Press, Oxford New York, pp 11–61
Stein PJ, Brammer JD, Ostroy SE (1979) Renewal of opsin in the photoreceptor cells of the mosquito. J Gen Physiol 74:565–582
Stowe S (1980) Rapid synthesis of photoreceptor membrane and assembly of new microvilli in a crab at dusk. Cell Tissue Res 211:419–440
Stowe S (1981) Effects of illumination changes on rhabdom synthesis in a crab. J Comp Physiol 142:19–25
Stowe S, de Couet H-G, Davis D (1990) Photoreceptor membrane turnover in the crayfish Cher ax destructor: electron microscopy and anti-rhodopsin electronmicroscopic immunocytochemistry. Cell Tissue Res 262:483–499
Suzuki T, Makino-Tasaka M, Eguchi E (1984) 3-dehydroretinal (vitamin A2 aldehyde) in crayfish eye. Vision Res 24:783–787
Uitto A, Sarvala J (1991) Seasonal growth of the benthic aphipods Pontoporeia affinis and P. femorata in a Baltic archipelago in relation to environmental factors. Mar Biol 111:237–246
Waldron KD (1953) A new subspecies of Pontoporeia affinis in Lake Washington, with a discussion of its morphology and life cycle. M.S. Thesis, University of Washington, USA
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Donner, K.O., Langer, H., Lindström, M. et al. Visual pigment, dark adaptation and rhodopsin renewal in the eye of Pontoporeia affinis (Crustacea, Amphipoda). J Comp Physiol A 174, 451–459 (1994). https://doi.org/10.1007/BF00191711
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191711