Abstract
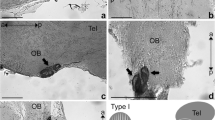
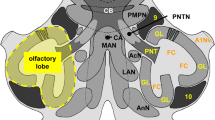
The olfactory bulb of many elasmobranch fishes is morphologically subdivided into distinct units or sub-bulbs immediately adjacent to the olfactory epithelium. We investigated this morphological feature in two species of shark and one species of ray in order to understand its impact on the arrangement of the primary olfactory projections onto the bulb. Using anterograde tracing methods in vitro (biocytin) as well as in fixed tissue (DiI), we observed a direct segregated projection of the olfactory afferents onto the bulb. Application of tracers to the lateral part of the olfactory epithelium resulted in staining restricted to the this region of the bulb, whereas the same tracers applied to the medial part of the epithelium resulted in staining of the medial olfactory bulb. The sub-bulbs appear to be individual anatomical units that each receive input from the olfactory lamellae. Nissl and myelin staining as well as the Golgi method show that the cytoarchitecture of the sub-bulbs is not substantially different from that of other anamniotes. However, we did note the existence of two types of mitral cell, based on the morphology of their dendritic arborization. Type L cells exhibit a loose dendritic arborization, whereas type T cells are characterized by a dense, bush-like dendritic arborization. Both types of mitral cells lack basal dendrites.
Similar content being viewed by others
References
Adrian ED (1950) Sensory discrimination with some recent evidence from the olfactory organ. Br Med Bull 6:330–333
Andres KH (1970) Anatomy and ultrastructure of the olfactory bulb in fish, amphibia, reptiles, birds, and mammals. In: Ciba Foundation Symposium on Taste and Smell. London, pp 177–196
Astic L, Saucier D (1986) Anatomical mapping of the neuroepithelial projection to the olfactory bulb in the rat. Brain Res Bull 16:445–454
Astic L, Saucier D (1988) Topographical projections of the septal organ to the main olfactory bulb in rats: ontogenetic study. Dev Brain Res 42:297–303
Astic L, Saucier D, Holley A (1987) Topographical relationship between olfactory receptor cells and glomerular foci in the rat olfactory bulb. Brain Res 424:144–152
Bauchot R, Platel R, Ridet JM (1976) Brain-body weight relation-ships in Selachii. Copeia 1976:305–309
Compagno LJV (1988) Sharks of the order Carcharhiniforms. Princeton University Press
Costanzo RM, Mozell MM (1976) Electrophysiological evidence for a topographical projection of the nasal mucosa onto the olfactory bulb of the frog. J Gen Physiol 68:297–312
Daniel JF (1934) The elasmobranch fishes. University of California Press
Døving KB, Selset R, Thommesen G (1980) Olfactory sensitivity to bile acids in Salmonid fishes. Acta Physiol Scand pp 24–32
Dubois-Dauphin ME, Tribollet ME, Dreifuss JP (1981) Relations somatotopiques entre la muqueuse olfactive et le bulbe olfactif chez le triton. Brain Res 219:269–287
Duncan HJ, Nickell WT, Shipley MT, Gesteland RC (1990) Organisation of projections from olfactory epithelium to olfactory bulb in the frog, Rana pipiens. J Comp Neurol 299:299–311
Erickson JR, Caprio J (1984) The spatial distribution of ciliated and microvillous olfactory receptor neurons in the channel catfish is not matched by a differential specificity to amino-acid and bile stimuli. Chem Senses 9:127–141
Freeman WJ (1974) Topographic organization of primary olfactory nerve in cat and rabbit as shown by evoked potentials. Electroencephalogr Clin Neurophysiol 36:33–45
Graziadei PPC, Monti-Graziadei GA (1986) Principles of organization of the vertebrate olfactory glomerulus: an hypothesis. Neuroscience 19:1025–1035
Greer CA, Stewart WB, Kauer JS, Shepherd GM (1981) Topographical and laminar localization of 2-deoxyglucose uptake in rat olfactory bulb induced by electrical stimulation of olfactory nerves. Brain Res 217:279–293
Hodgson ES, Mathewson RF (1978) Electrophysiological studies of chemoreception in elasmobranchs. In: Hodgson ES, Mathewson RF (eds) Sensory biology of sharks, skates and rays. Office of Naval Research, Arlington, VA, pp 227–267
Iwahori N, Nakamura K, Mameya C (1989) A Golgi study on the main olfactory bulb in the snake Elaphe quadrivirginita. Neurosci Res 6:411–425
Jastreboff PJ, Pedersen PE, Greer CA, Stewart WB, Kauer JS, Benson TE, Shepherd GM (1984) Specific olfactory receptor populations projecting to identified glomeruli in the rat olfactory bulb. Proc Natl Acad Sci USA 81:5250–5254
Johnsen PB, Teeter JH (1985) Behavioral responses of bonnethead sharks (Sphyrna tiburo) to controlled olfactory stimulation. Mar Behav Physiol 11:283–291
Kauer JS (1987) Coding in the olfactory system. In: Finger TE, Silver WC (eds) Neurobiology of taste and smell. Wiley-Interscience, New York, pp 205–231
Kauer JS (1988) Real-time imaging of evoked activity in local circuits of the salamander olfactory bulb. Nature 331:166–168
Kauer JS (1991) Contribution of topography and parallel processing to odor coding in the vertebrate olfactory pathway. Trends Neurosci 14:79–85
Kauer JS, Moulton DG (1974) Responses of olfactory bulb neurones to odour stimulation of small areas in the salamander. J Physiol 243:717–737
Kleerekoper H (1978) Chemoreception and its interaction with flow and light perception in the locomotion and orientation of some elasmobranchs. In: Hodgson ES, Mathewson RF (eds) Sensory biology of sharks, skates and rays. Office of Naval Research, Arlington, VA, pp 270–329
Lancet D, Greer CA, Kauer JS, Shepherd GM (1982) Mapping of odor-related neuronal activity in the olfactory bulb by high resolution 2-deoxyglucose autoradiography. Proc Natl Acad Sci USA 79:670–674
Land LJ, Shepherd GM (1974) Autoradiographic analysis of olfactory receptor projections. Brain Res 70:506–510
Le Gros Clark WE (1951) The projections of the olfactory epithelium on the olfactory bulb in the rabbit. J Neurol Neurosurg Psychiatry 14:1–10
Mackay-Sim A, Nathan MH (1984) The projection from the olfactory epithelium to the olfactory bulb in the salamander, Ambystoma tigrinum. Anat Embryol 170:93–97
Norris HW, Hughes SP (1920) The cranial, occipital, and anterior spinal nerves of the dogfish, Sphyrna acanthias. J Comp Neurol 31:293–400
Northcutt RG (1978) Brain organization in the cartilaginous fishes. In: Hodgson ES, Mathewson RF (eds) Sensory biology of sharks, skates and rays. Office of Naval Research, Arlington, VA, pp 117–193
Oka Y (1983) Golgi, electron microscopic and combined Golgielectron microscopic studies of the mitral cells in the goldfish olfactory bulb. Neuroscience 8:723–742
Riddle D, Oakley B (1991) Evaluation of projection patterns in the primary olfactory system of rainbow trout. J Neurosci 11 (12):3752–3762
Satou M, Fujita I, Ichikawa M, Yamaguchi K, Ueda K (1983) Field potential and intracellular potential studies of the olfactory bulb in the carp: evidence for a functional separation of the olfactory bulb into lateral and medial subdivisions. J Comp Physiol 152:319–333
Scalia F, Gallousis G, Roca S (1991) A note on the organization of the amphibian olfactory bulb. J Comp Neurol 305:435–442
Shepherd GM (1981) The olfactory glomerulus: its significance for sensory processing. In: Katsuki Y, Norgren R, Sato M (eds) Brain mechanisms of sensation. Wiley, New York, pp 209–223
Sicard G, Royet JP, Jourdan F (1989) A comparative study of the 2-deoxyglucose patterns of glomerular activation in the olfactory bulb of C57BL/6J and AKR/J mice. Brain Res 481:325–334
Sterzi G (1909) Il systema nervoso centrale dei vertebrati, vol 2, pt.1. Draghi, Padova
Thommessen G (1977) Spatial differences in specificity of trout (Sphyrna trutta L.) olfactory bulb. Acta Physiol Scand 96:6A-7A
Thommessen G (1978) The spatial distribution of odour-induced potentials in the olfactory bulb of char and trout (Salmonidae). Acta Physiol Scand 102:205–217
Thommessen G (1982) Specificity and distribution of receptor cells in the olfactory mucosa of char (Sphyrna alpinus). Acta Physiol Scand 115:47–56
Thommessen G (1983) Morphology, distribution and specificity of olfactory receptor cells in salmonid fishes. Acta Physiol Scand 117:241–249
Zielinski B, Hara TJ (1988) Morphological and physiological development of olfactory receptor cells in rainbow trout (Salmo gairdneri) embryos. J Comp Neurol 271:300–311
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dryer, L., Graziadei, P.P.C. A pilot study on morphological compartmentalization and heterogeneity in the elasmobranch olfactory bulb. Anat Embryol 188, 41–51 (1993). https://doi.org/10.1007/BF00191450
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191450