Abstract
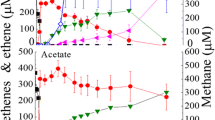
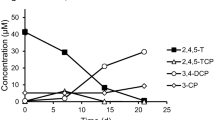
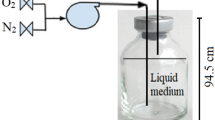
The transformations of 14CCl4 by whole cells of Acetobacterium woodii suspended in phosphate buffer containing reducing agents, and by the cobalt corrinoid aquocobalamin in the same solution, were compared. Each catalyst transformed 14CCl4 not only to reduced products (CHCl3 and CH2Cl2) but also to CO and CO2 as well as non-volatile products. The mass balance for radioactive carbon was complete in each case. Thus, the reactions of the pure cobalt corrinoid resemble the reactions in vivo. The proton in CHCl3, formed from CCl4 by A. woodii, was derived from water. Extracts of A. woodii were fractionated into large and small molecules, and each of the two fractions was separated chromatographically. Fractions of proteins demonstrated poor correlation between content of the corrinoid vitamin B12 and rates of transformation of CCl4. The correlation was somewhat improved if the fractions were autoclaved, but dechlorination in the absence of vitamin B12 was observed. Separation of the small molecules yielded only one fraction containing vitamin B12, and this fraction catalyzed dechlorination, whereas several other fractions were able to dechlorinate CCl4 in the absence of vitamin B12. We presume there to be unrecognized dechlorinative factors in anaerobic bacteria.
Similar content being viewed by others
Abbreviations
- GC:
-
gas chromatograph(y)
- GC-MS (or-TCD or-FID):
-
GC coupled to a mass spectrometer (or a thermal conductivity detector or a flame ionization detector)
- HPLC:
-
high pressure liquid chromatograph(y)
- FPLC:
-
high pressure protein chromatograph(y)
References
AhrHJ, KingLJ, NastainczykW & UllrichV (1980) The mechanism of chloroform and carbon monoxide formation from carbon tetrachloride by microsomal cytochrome P-450. Biochem. Pharmacol. 29: 2855–2861
BalchWE, FoxGE, MagrumLJ, WoeseCR & WolfeRS (1979) Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43: 260–296
BalchWE, SchoberthS, TannerRS & WolfeRS (1977) Acetobacterium, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria. Int. J. Syst. Bacteriol. 27: 355–361
BouwerEJ & McCartyPL (1983) Transformations of halogenated organic compounds under denitrification conditions. Appl. Environ. Microbiol. 45: 1295–1299
BradfordM (1976) A rapid and sensitive method for the quantitation of microgram amounts of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
CookAM, ScholtzR & LeisingerT (1988) Mikrobieller Abbau von halogenierten aliphatischen Verbindungen. GWF Gas Wasserfach: Wasser Abwasser, 129: 61–69
CriddleCS, DeWittJT, Grbic-GalicD & McCartyPL (1990a) Transformation of carbon tetrachloride by Pseudomonas sp. strain KC under denitrifying conditions. Appl. Environ. Microbiol. 56: 3240–3246
CriddleCS, DeWittJT & McCartyPL (1990b) Reductive dehalogenation of carbon tetrachloride by Escherichia coli K-12. Appl. Environ. Microbiol. 56: 3247–3254
DangelW, SchulzH, DiekertG, KönigH & FuchsG (1987) Occurrence of corrinoid-containing membrane proteins in anaerobic bacteria. Arch. Microbiol. 148: 52–56
EgliC, ScholtzR, CookAM & LeisingerT (1987) Anaerobic dechlorination of tetrachloromethane and 1,2-dichloroethane to degradable products by pure cultures of Desulfobacterium sp. and Methanobacterium sp. FEMS Microbiol. Lett. 43: 257–261
EgliC, StromeyerS, CookAM & LeisingerT (1990) Transformation of tetra- and trichloromethane to CO2 by anaerobic bacteria is a non-enzymic process. FEMS Microbiol. Lett. 68: 207–212
EgliC, ThüerM, SuterD, CookAM & LeisingerT (1989) Mono- and dichloroacetic acids as carbon and energy sources for a stable, methanogenic mixed culture. Arch. Microbiol. 152: 218–223
EgliC, TschanT, ScholtzR, CookAM & LeisingerT (1988) Transformation of tetrachloromethane to dichloromethane and carbon dioxide by Acetobacterium woodii. Appl. Environ. Microbiol. 54: 2819–2824
FridrichW (1975) Vitamin B12 und verwandte Corrinoide. Georg Thieme Verlag, Stuttgart
FreedmanDL & GossettJM (1991) Biodegradation of dichloromethane and its utilization as a growth substrate under methanogenic conditions. Appl. Environ. Microbiol. 57: 2847–2857
FuchsG, StupperichE & EdenG (1980) Autotrophic CO2 fixation in Chlorobium limicola. Evidence for the operation of a reductive tricarboxylic acid cycle in growing cells. Arch. Microbiol. 128: 64–71
GälliR & McCartyPL (1989) Biotransformation of 1,1,1-trichloroethane, trichloromethane, and tetrachloromethane by a Clostridium sp. Appl. Environ. Microbiol. 55: 837–844
GantzerCJ & WackettPL (1991) Reductive dechlorination catalyzed by bacterial transition-metal coenzymes. Environ. Sci. Technol. 25: 715–722
GrossenbacherH, ThurnheerT, ZürrerD & CookAM (1986) Determination of sulfonated azo dyestuffs and their bacterial metabolites by high pressure liquid chromatography. J. Chromatogr. 360: 219–223
HolligerC, SchraaG, StamsAJM & ZehnderAJB (1990) Reductive dechlorination of 1,2-dichloroethane and chloroethane by cell suspensions of methanogenic bacteria. Biodegradation 1: 253–261
KennedySIT & FewsonCA (1968) Enzymes of the mandelate pathway in bacterium N.C.I.B. 8250. Biochem. J. 107: 497–506
KinerA & LeisingerT (1983) Oxygen sensitivity of methanogenic bacteria. Syst. Appl. Microbiol. 4: 305–312
KleckaGM & GonsiorSJ (1984) Reductive dechlorination of chlorinated methanes and ethanes by reduced iron(II) porphyrins. Chemosphere 13: 391–402
KroneUE, LauferK, ThauerRK & HogenkampHPC (1989a) Coenzyme F430 as a possible catalyst for the reductive dehalogenation of chlorinated C1-hydrocarbons in methanogenic bacteria. Biochemistry 28: 10061–10065
KroneUE, ThauerRK & HogenkampHPC (1989b) Reductive dehalogenation of chlorinated C1-hydrocarbons mediated by corrinoids. Biochemistry 28: 4908–4914
KroneUE, ThauerRK, HogenkampHPC & SteinbachK (1991) Reductive formation of carbon monoxide from CCl4 and FREONS 11, 12, and 13 catalyzed by corrinoids. Biochemistry 30: 2713–2719
KuhnEP & SuflitaJM (1989) Dehalogenation of pesticides by anaerobic microorganisms in soils and groundwater—a review. SSSA Spec. Publ. 22: 111–180
LocherHH, MalliC, HooperSW, VorherrT, LeisingerT & CookAM (1991) Degradation of p-toluic acid (p-toluene carboxylic acid) and p-toluene sulphonic acid via oxygenation of the methyl sidechain is initiated by the same set of enzymes in Comamonas testosteroni T-2. J. Gen. Microbiol. 137: 2201–2208
LuriaSE (1960) The bacterial protoplasm: composition and organization. In: GunsalusIC & StanierRY (Eds.), The Bacteria, vol 1. (pp 1–34). Academic Press, New York
ScholtzR, SchmuckleA, CookAM & LeisingerT (1987) Degradation of eighteen 1-monohaloalkanes by Arthrobacter sp. strain HA1. J. Gen. Microbiol. 133: 267–274
StromeyerSA, WinkelbauerW, KohlerH, CookAM & LeisingerT (1991) Dichloromethane utilized by an anaerobic mixed culture: acetogenesis and methanogenesis. Biodegradation 2: 129–137
Stumpf K (1990) Reduktive und substitutive Dechlorierung von CCl4 bei Anaerobiern. Semesterarbeit, ETH-Zürich
StupperichE, EisingerHJ & KräutlerB (1988) Diversity of corrinoids in acetogenic bacteria: p-cresolylcobalamide from Sporomusa ovata, 5-methoxy-6-methylbenzimidazolylcobamide from Clostridium formicoaceticum and vitamin B12 from Acetobacterium woodii. Eur. J. Biochem. 172: 459–464
StupperichE & KräutlerB (1988) Pseudo vitamin B12 or 5-hydroxybenzimidazolylcobamide are the corrinoids found in methanogenic bacteria. Arch. Microbiol. 149: 268–271
TrauneckerJ, PreußA & DiekertG (1991) Isolation and characterization of a methyl chloride utilizing, strictly anaerobic bacterium. Arch. Microbiol. 156: 416–421
VogelTM, CriddleCS & McCartyPL (1987) Transformations of halogenated aliphatic compounds. Environ. Sci. Technol. 21: 722–736
WackettPL (1991) Dehalogenation reactions catalyzed by bacteria. In: MartinAM (Ed), Biological Degradation of Waste (pp 187–203). Elsevier, Essex
WelzB (1983) Atomabsorptionsspektrometrie, 3rd Ed. Verlag Chemie, Weinheim
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stromeyer, S.A., Stumpf, K., Cook, A.M. et al. Anaerobic degradation of tetrachloromethane by Acetobacterium woodii: separation of dechlorinative activities in cell extracts and roles for vitamin B12 and other factors. Biodegradation 3, 113–123 (1992). https://doi.org/10.1007/BF00189639
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00189639