Abstract
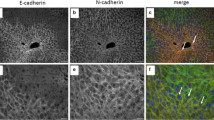
Sequential changes of gap junctions (GJs), tight junctions (TJs) and desmosomes (DSs) between hepatocytes during restorative proliferation were studied in rats after a single intraperitoneal administration of 200 mg/kg thioacetamide (TAA). Antibody against connexin 32 was used to demonstrate GJs; simultaneously the changes in TJs and DSs were studied using antibodies against 7H6 protein and desmoplakins. Propidium iodide and bromodeoxyuridine were used to recognize necrotic and proliferative cells. GJs were evenly distributed in early necrotic hepatocytes at 16 h after TAA treatment, then disappeared from necrotic and surrounding cells at 24 h. At 48 h, GJs had disappeared completely from hepatocytes in whole liver lobules, while many hepatocytes were heavily labelled with BrdU. At 72 h, GJs reappeared, firstly in perinecrotic areas. At 96 h after treatment, when the injured areas had disappeared and restorative proliferation ceased, GJs were distributed evenly throughout the lobules. Immunohistochemical observation of GJs in centrilobular, perinecrotic and periportal areas after TAA-induced hepatic necrosis was confirmed by counting the number of connexin-32-positive spots in the respective areas. TJs and DSs disappeared from necrotic cells at 24 h, but then increased between 24 and 48 h in perinecrotic areas, though the increased intensity of these junctions was more evident at 48 h. At 72 h, localization of TJs and DSs returned to normal. These results suggest that during the course of acute hepatic injury, GJs (cell-cell communication) behave differently from other intercellular junctions.
Similar content being viewed by others
References
Fallon MB, Mennone A, Anderson JM (1993) Altered expression and localization of the tight junction protein ZO-1 after common bile duct ligation. Am J Physiol 264:C1439-C1447
Garrod DR (1987) Desmosome: cell adhesion molecules and the adhesive properties of cells in tissues. J Cell Sci 4:221–237
Ledda-Columbano GM, Coni M, Curto M, Giacomini L, Fass G, Oliverio S, Placentini M, Columbano A (1991) Induction of two different modes of cell death apoptosis and necrosis in rat liver after a single dose of thioacetamide. Am J Pathol 139:1099–1109
Mandel LJ, Bacallao R, Zampighi G (1993) Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature 361:552–555
Mensil M, Fitzgerald DJ, Yamasaki H (1988) Phenobarbital specifically reduces gap junction protein mRNA level in rat liver. Mol Carcinog 1:79–81
Metz J, Bressler D (1979) Reformation of gap and tight junctions in regenerating liver after cholestasis. Cell Tissue Res 199:257–270
Metz J, Aoki A, Merlo M, Forrsmann WG (1977) Morphological alterations and functional changes of interhepatocellular junctions induced by bile duct ligation. Cell Tissue Res 182:299–310
Miyasita T, Takeda A, Iwai M, Shimazu T (1991) Single administration of hepatotoxic chemicals transiently decreases the gap-junction-protein levels of connexin 32 in rat liver. Eur J Biochem 196:37–42
Robenek H, Themann H (1979) Effect of chronic administration of thioacetamide (TAA) on the structure of bile canaliculi and tight junctions in the rat liver as revealed by freeze-fracturing. Virchows Arch [B] 32:57–67
Sáez JC, Spray DC, Nairn AC, Hertzberg EL, Greengard P, Bennett MVL (1986) c-AMP increases junctional conductance and stimulates phosphorylation of the 27 kDa principal gap junction polypeptide. Proc Natl Acad Sci USA 83:2473–2476
Sáez JC, Bennett MVL, Spray DC (1987) Carbon tetrachloride at hepatotoxic levels blocks reversibly gap junctions between rat hepatocytes. Science 236:967–969
Sáez JC, Conner JA, Spray DC, Bennett MVL (1989) Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-triphosphate and to calcium ions. Proc Natl Acad USA 86:2708–2712
Sáez JC, Spray DC, Hertzberg EL (1990) Gap junctions: biochemical properties and functional regulation under physiological and toxicological conditions. In Vitro Toxicol 3:69–86
Sakamoto H, Oyamada M, Enomoto K, Mori M (1992) Differential changes in expression of gap junction proteins connexin 26 and 32 during hepatocarcinogenesis in rats. Jpn J Cancer Res 83:1210–1215
Spray DC, Sáez JC, Hertzberg EL (1988) Gap junctions between hepatocytes: structural and regulatory features. In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA (eds) The liver: biology and pathobiology, 2nd edn. Raven Press, New York, pp 851–866
Staehelin LA, Hull BE (1979) Junctions between living cells. Sci Am 238:140–153
Suematu M, Kato S, Ishii H, Asaka H, Yanagisawa T, Suzuki H, Oshio C, Tsuchiya M (1991) Intralobular heterogeneity of carbon tetrachloride-induced oxidative stress in perfused rat liver visualized by digital imaging fluorescence microscopy. Lab Invest 64:167–173
Sugie S, Mori H, Takahashi M (1987) Effect of in vivo exposure to the liver tumor promoters phenobarbital or DDT on the gap junctions of rat hepatocytes: a quantitative freeze-fracture analysis. Carcinogenesis 8:45–51
Traub O, Druge PM, Willecke K (1983) Degradation and resynthesis of gap junction protein in plasma membrane of regenerating liver after partial hepatectomy or cholestasis. Proc Natl Acad Sci USA 80:755–759
Vos RD, Desmet VJ (1978) Morphologic changes of the junctional complex of the hepatocytes in rat liver after bile duct ligation. Br J Pathol 59:220–227
Watanabe N, Tsukada N, Smith CR, Philips MJ (1991) Motility of bile canaliculi in the living animal: implications for bile flow. J Cell Biol 113:1069–1080
Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M (1993) Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J Cell Biol 120:477–483
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kojima, T., Sawada, N., Zhong, Y. et al. Sequential changes in intercellular junctions between hepatocytes during the course of acute liver injury and restoration after thioacetamide treatment. Vichows Archiv A Pathol Anat 425, 407–412 (1994). https://doi.org/10.1007/BF00189579
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00189579