Abstract
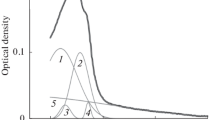
The interaction of recombinant human epidermal growth factor with small unilamellar phospholipid vesicles was studied by steady-state and time-resolved fluorescence of the bis-tryptophan sequence (Trp49-Trp50). Steady-state anisotropy measurements demonstrate that strong binding occurred with small unilamellar vesicles made up of acidic phospholipids at acidic pH only (pH≤ 4.7). An apparent stoichiometry for 1,2-dimyristoyl-sn-phosphoglycerol of about 12 phospholipid molecules per molecule of human epidermal growth factor was estimated. The binding appears to be more efficient at temperatures above the gel to liquid-crystalline phase transition. The conformation and the environment of the Trp-Trp sequence are not greatly modified after binding, as judged from the invariance of the excited state lifetime distribution and from that of the fast processes affecting the anisotropy decay. This suggests that the Trp-Trp sequence is not embedded within the bilayer, in contrast to the situation in surfactant micelles (Mayo et al. 1987; Kohda and Inigaki 1992).
Similar content being viewed by others
Abbreviations
- DMPG:
-
1,2-dimyristoyl-sn-phosphoglycerol
- hEGF:
-
human Epidermal Growth Factor
- HPLC:
-
high performance liquid chromatography
- MEM:
-
Maximum Entropy Method
- POPC:
-
1-palmitoyl, 2-oleoyl-sn-phosphocholine
- POPS:
-
1-palmitoyl, 2-oleoyl-sn-phosphoserine
- SUV:
-
small unilamellar vesicles
- Trp:
-
tryptophan
- Trp-Trp:
-
bis-tryptophan
References
Bastiaens PH, Bonants PJM Müller F, Visser AJWG (1989) Timeresolved fluorescence spectroscopy of NADPH-cytochrome P-450 reductase: demonstration of energy transfer between the two prosthetic groups. Biochemistry 28:8416–8425
Besada V, Antuch W, Cinza A, Rojas I, Quintana M, Padrón G (1990) Chemical characterization of recombinant human epidermal growth factor. Anal Chim Acta 235:301–305
Carpenter G (1987) Receptors for epidermal growth factor and other polypeptide mitogens. Ann Rev Biochem 56:881–914
Carpenter G, Cohen S (1990) Epidermal growth factor. J Biol Chem 265:7709–7712
Cohen S (1962) Isolation of a mouse submaxillary protein accelerating incisor eruption and eyelid opening in the new-born animals. J Biol Chem 237:1555–1562
Cooke RM, Wilkinson AJ, Baron M, Pastore A, Tappin MJ, Campbell ID, Gregory H, Sheard B (1987) The solution structure of human epidermal growth structure. Nature 327:339–341
Datema KP, Visser AJWG, van Hoek A, Wolfs CJAM, Spruijt RB, Hemminga MA (1987) Time-resolved tryptophan fluorescence anisotropy investigation of bacteriophage M13 coat protein in micelles and in mixed bilayers. Biochemistry 26:6145–6152
Gentin M, Vincent M, Brochon JC, Livesey AK, Cittanova N, Gallay J (1990) Time-resolved fluorescence of the single tryptophan residue in rat α-fetoprotein and rat serum albumin: analysis by the maximum entropy method. Biochemistry 29:10405–10412
Greenfield C, Hiles I, Waterfield MD, Federwisch M, Wollmer A, Blundell TL, McDonald N (1989) Epidermal growth factor binding induces a conformational change in the external domain of its receptor. EMBO J 8:4115–4123
Harnois I, Genest D, Brochon JC (1988) Micellization and interactions with phospholipid vesicles of the lipopeptide Iturin A, as monitored by time-resolved fluorescence of a D-tyrosyl residue. Biopolymers 27:1403–1413
Holladay LA, Savage CR, Cohen S, Puett D (1976) Conformation and unfolding thermodynamics of epidermal growth factor and derivatives. Biochemistry 15:2624–2633
John E, Jähnig F (1988) Dynamics of melittin in water and membrane as determined by fluorescence anisotropy decay. Biophys J 54:817–827
Kohda D, Inagaki F (1992) Structure of epidermal growth factor bound to perdeuterated dodecylphosphocholine micelles determined by two-dimensional NMR and simulated annealing calculations. Biochemistry 31:677–685
Kuipers OP, Vincent M, Brochon JC, Verheij HM, de Haas GH, Gallay J (1991) Insight into the conformational dynamics os specific regions of porcine pancreatic phospholipase A2 from a time-resolved fluorescence study of a genetically inserted single tryptophan residue. Biochemistry 30:8771–8785
Livesey AK, Brochon JC (1987) Analyzing the distribution of decay constants in pulse-fluorimetry using the Maximum Entropy Method Biophys J 52:693–706
Makino K, Morimoto M, Nishi M, Sakamoto S, Tamura A, Inooka H, Asaka K (1987) Proton nuclear magnetic resonance study on the solution conformation of human epidermal growth factor. Proc Natl Acad Sci USA 84, 7841–7845
Mayo KH, De Marco A, Menegatti E, Kaptein R (1987) Interaction of epidermal growth factor with micelles monitored by photochemically induced dynamic nuclear polarization-1H NMR spectroscopy. J Biol Chem 262:14899–14904
Mayo KH, Cavalli RC, Peters AR, Boelens R, Kaptein R (1989) Sequence specific 1H-NMR assignments and peptide backbone conformation in rat epidermal growth factor. Biochem J 257:197–205
Merola F, Rigler R, Holmgren A, Brochon JC (1989) Picosecond tryptophan fluorescence of thioredoxin: evidence for discrete species in slow exchange. Biochemistry 28:3383–3398
Montelione GT, Wüthrich K, Burgess AW, Nice EC, Wagner G, Gibson KD, Scheraga HA (1992) Solution structure of epidermal growth factor determined by NMR spectroscopy and refined energy minimization with restraints. Biochemistry 31:236–249
Moy FJ, Scheraga HA, Liu J-F, Wu R, Montelione GT (1989) Conformational characterization of a single-site mutant of murine epidermal growth factor (EGF) by 1H NMR provides evidence that leucine-47 is involved in the interactions with the EGF receptor. Proc Natl Acad Sci USA 86:9836–9840
Perrin F (1926) Polarisation de la lumière de fluorescence. Vie moyenne des molécules dans l'état excité. J de Physique 7:390–401
Sargent DF, Schwyzer R (1986) Membrane lipid phase as catalyst for peptide-receptor interactions. Proc Natl Acad Sci USA 83:5774–5778
Strickland EH, Billups C, Kay E (1972) Effects of hydrogen bonding and solvents upon the tryptophanyl 1La absorption band. Studies using 2,3-dimethylindole. Biochemistry 19:3657–3662
Tanford C, Reynolds JA (1976) Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta 457:133–170
Taylor JM, Mitchell WM, Cohen S (1972) Epidermal growth factor, physical properties. J Biol Chem 247:5928–5934
van Dijck PWM, de Kruijff B, Verkleij AJ, van Deenen LLM, de Gier J (1978) Comparative study on the effects of pH and Ca" on bilayers of various negatively charged phospholipids and their mixture with phosphatidylcholine. Biochim Biophys Acta 512:84–96
Vincent M, de Foresta B, Gallay J, Alfsen A (1982) Nanosecond fluorescence anisotropy decay of n-(9-anthroyloxy) fatty acids in dipalmitoyl phosphatidylcholine vesicles with regards to isotropic solvents. Biochemistry 21:708–716
Vincent M, Brochon JC, Merola F, Jordi W, Gallay J (1988) Nanosecond dynamics of horse heart apocytochrome c in aqueous solution as studied by time-resolved fluorescence of the single tryptophan residue (Trp-59). Biochemistry 27:8752–8761
Vincent M, Gallay J (1991) The interactions of horse heart apocytochrome c with phospholipid vesicles and surfactant micelles: time-resolved fluorescence study of the single tryptophan residue (Trp-59). Eur Biophys J 20:183–191
Vogel H, Nilsson L, Rigler R, Voges KP, Jung G (1988) Structural fluctuations of a helical polypeptides traversing a lipid bilayer. Proc Natl Acad Sci USA 85:5067–5071
Wahl PH (1979) Analysis of fluorescence anisotropy decay by least square method. Biophys Chem 10:91–104
Weber G, Shinitzki M (1972) Failure of energy transfer between identical aromatic molecules on excitation at the long wave edge of the absorption spectrum. Proc Natl Acad Sci USA 65:8723–8730
Yarden Y, Ullrich A (1988) Growth factors receptor tyrosine kinases. Ann Rev Biochem 57:443–478
Yguerabide J (1972) Nanosecond spectroscopy of macromolecules. Methods in Enzymology 26 part C:498–578
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li De La Sierra, I.M., Vincent, M., Padron, G. et al. Interaction of recombinant human epidermal growth factor with phospholipid vesicles. A steady-state and time-resolved fluorescence study of the bis-tryptophan sequence (TRP49-TRP50). Eur Biophys J 21, 337–344 (1992). https://doi.org/10.1007/BF00188346
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00188346