Summary
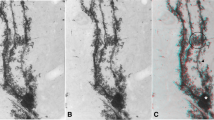
Bergmann glial (Golgi epithelial) cells were Golgi-impregnated in the cerebella of species with great differences in the thickness of the molecular layer, in small African native mouse, rat, rhesus monkey, and man. The thickness of the molecular layer determines the length of the radial Bergmann cell processes. Whereas the overall morphology of the cells was found to be strikingly similar in all species studied, there were great quantitative differences in length and diameter of the stem processes. Species with thick molecular layers (man, monkey) have thicker stem processes than species with short distances between Bergmann glial cell soma and pial surface (rat, mouse). This could mean that larger animals with longer gestation periods allow for prolonged growth of cell volumes. On the other hand, an increase in the diameter of long processes should reduce the cytoplasmic resistance against ionic currents; this would be important when Bergmann glial cells — like retinal Müller cells — would act as “cables” for spatial buffering of potassium ions released by electrically active neurons. By contrast, the fractal dimension — i.e., a quantitative measure of the complexity of the cell's border of the cell processes was lower in species with long processes. In an age series of rat cells, the fractal dimension is shown to increase slightly up to a very old age.
Similar content being viewed by others
References
Braitenberg V, Guglielmotti V, Sada E (1967) Correlation of crystal growth with the staining of axons by the Golgi procedure. Stain Technol 42:277–283
Flook AG (1978) The use of dilation logic on the quantimet to achieve fractal dimension characterisation of textured and structured profiles. Powder Technol 21:295–298
Futamachi KJ, Mutani R, Prince DA (1974) Potassium activity in rabbit cortex. Brain Res 75:5–25
Gardner-Medwin AR (1984) A foot in the vitreous fluid. Nature 309:113
Hanke S, Reichenbach A (1987) Quantitative-morphometric aspects of Bergmann glial (Golgi epithelial) cell development in rats. Anat Embryol 177:183–188
Harris RM (1985) Light microscopic depth measurements of thick sections. J Neurosci Methods 14:97–100
Kocsis JD (1986) Functional characteristics of potassium channels of normal and pathological mammalian axons. In: Richtie JM, Keynes RD, Bolis R (eds) Neurology and neurobiology, vol 20, Ion channels in Neural Membranes. Liss, New York, pp 123–144
Lange W (1976) The myelinated parallel fibres of the cerebellar cortex and their regional distribution. Cell Tissue Res 166:489–496
Mandelbrot BB (1982) The fractal geometry of nature. Freeman, New York
Mugnaini E (1972) The histology and cytology of the cerebellar cortex. In: Larsell O, Jansen J (eds) The comparative anatomy and histology of the cerebellum. University of Minnesota Press, Minnesota, pp 201–264
Mutani R, Futamachi KJ, Prince DA (1974) Potassium activity in immature cortex. Brain Res 75:27–39
Newman EA (1984) Regional specialization of retinal glial cell membrane. Nature 309:155–157
Newman EA (1986) High potassium conductance in astrocyte end-feet. Science 223:453–454
Newman EA, Frambach DA, Odette LL (1984) Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 225:1174–1175
Pouwels E (1978) On the development of the cerebellum of the trout, Salmo gairdeneri. V. Neuroglial cell development Anat Embryol 3:67–83
Rall W (1977) Core conductor theory and cable properties of neurons. In: Brookhart JM, Mountcastle VB (eds) Handbook of physiology, Sect 1: The Nervous System, vol 1, Cellular biology of neurons, Part 1. Am Physiol Soc, Bethesda, pp 39–97
Ramon y Cajal S (1955) Histologie du Système Nerveux de l'Homme et des Vertébrés. CSIC Madrid (Reprint of 1909–1911 edn)
Reichelt W, Dettmer D, Brückner G, Brust P, Eberhardt W, Reichenbach A (1989) Potassium as a signal for both proliferation and differentation of rabbit retinal (Müller) glial cells growing in cell culture. Cell Signaling 1:187–194
Reichenbach A (1989) Attempt to classify glial cells by means of their process specialization using the rabbit retinal Müller cells as an example of cytotopographic specialization of glial cells. Glia 2:250–259
Reichenbach A, Reichelt W (1986) Postnatal development of radial glial (Müller) cells of the rabbit retina. Neurosci Lett 71:125–130
Reichenbach A, Neumann M, Brückner G (1987) Cell length to diameter relation of rat fetal radial glia — does impaired K+ transport capacity of long thin cells cause their perinatal transformation into multipolar astrocytes? Neurosci Lett 73:95–100
Reichenbach A, Schneider H, Leibnitz L, Reichelt W, Schaaf P, Schümann R (1989) The structure of rabbit retinal Müller (glial) cells is adapted to the surrounding retinal layers. Anat Embryol 180:71–79
Sacher GA, Staffelt EF (1974) Relation of gestation time to brain weight for placental mammals: implications for the theory of vertebrate growth. Am Nat 108:593–615
Senitz D, Goertchen R (1978) Über Astrozytenveränderungen in der orbitofrontalen Hirnrinde bei seniler Demenz. Zentralbl Allg Pathol 122:515–521
Smith TG Jr, Marks WB, Lange GD, Sheriff WH Jr, Neale EA (1989) A fractal analysis of cell images. J Neurosci Methods 27:173–180
Smith TG Jr, Behar TN, Lange GD, Marks WB, Sheriff WH Jr (1991) A fractal analysis of cultured rat optic nerve glial growth and differentiation. Neuroscience 41:159–169
Somjen GG (1978) Metabolic and electrical correlates of the clearing of excess potassium in the cortex and spinal cord. In: Porter A (ed) Studies in neurophysiology. Cambridge University Press, Cambridge, pp 181–201
Stolzenburg J-U, Reichenbach A, Neumann M (1989) Size and density of glial and neuronal cells within the cerebral neocortex of various insectivorian species. Glia 2:78–84
Syková E (1981) Extracellular K+ accumulation in the spinal cord. In: Syková E, Hnik P, Vyklicky L (eds) Ion-selective microelectrodes and their use in excitable tissues. Plenum Press, New York London, pp 139–158
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Siegel, A., Reichenbach, A., Hanke, S. et al. Comparative morphometry of Bergmann glial (Golgi epithelial) cells. Anat Embryol 183, 605–612 (1991). https://doi.org/10.1007/BF00187909
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00187909