Abstract
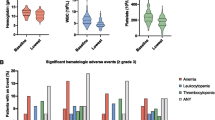
Rhenium-186 hydroxyethylidene diphosphonate (186Re-HEDP) has been used for the palliative treatment of metastatic bone pain. A phase 1 dose escalation study was performed using 186Re-HEDP Twenty-four patients with hormone-resistant prostate cancer entered the study. Each patient had at least four bone metastases and adequate haematological function. Groups of at least three consecutive patients were treated with doses starting at 1295 MBq and increasing to 3515 MBq (escalated in increments of 555 MBq). Thrombocytopenia proved to be the dose-limiting toxicity, while leucopenia played a minor role. Early death occurred in one patient (10 days after administration) without clear relationship to the 186Re-HEDP therapy. Transient neurological dysfunction was seen in two cases. Two patients who received 3515 MBq 186Re-HEDP showed grade 3 toxicity (thrombocytes 25–50 × 109/1), defined as unacceptable toxicity. After treatment alkaline phosphatase levels showed a transient decrease in all patients (mean: 26% ± 10% IUA; range: 11%–44%). Prostate-specific antigen values showed a decline in eight patients, preceded by a temporary increase in three patients. From this study we conclude that the maximally tolerated dose of 186Re-HEDP is 2960 MBq. A placebo-controlled comparative study on the efficacy of 186Re-HEDP has been initiated.
Similar content being viewed by others
References
Jacobs SC. Spread of prostatic cancer to bone. Urology 1983; 21:337–344.
Hendrickson FR, Shehata WM, Kirchner AB. Radiation therapy for osseous metastasis. Int J Radiat Oncol Biol Phys 1976; 1: 275–278.
Gilbert HA, Kagan AR, Nussbaum H, Rao AR, Satzman J, Chan P, Allen B, Forsythe A. Evaluation of radiation therapy for bone metastases: pain relief and quality of life. Am J Roentgenol 1977; 129: 1095–1096.
Kuban DA, Delbridge T, El-Mahdi AM, Schellhammer PF Half-body irradiation for treatment of widely metastatic adenocarcinoma of the prostate. J Urol 1989; 141: 572–574.
Fitzpatrick PJ, Rider WD. Half body radiotherapy. Int J Radiat Oncol Biol Phys 1976; 1: 197–207.
Wilkins MF, Keen CW. Hemi-body radiotherapy in the management of metastatic carcinoma. Clin Radiol 1987; 38: 267–268.
Hoskin PJ. Radiotherapy in the management of bone metastases. In: Rubens RD, Fogelman I, eds. Bone metastases: diagnosis and treatment. London: Springer; 1991: 171–185.
Hoskin PJ. Palliation of bone metastases. Eur J Cancer 1991; 27:950–951.
Poulsen HS, Nielsen OS, Klee M, Rorth M. Palliative irradiation of bone metastases. Cancer Treat Rev 1989; 16: 41–48.
Perez CA, Cosmatos D, Carcia DM, Eisbruch AE, Poulter CA. Irradiation in relapsing carcinoma of the prostate. Cancer 1993; 71: 1110–1122.
Lewington VJ. Targeted radionuclide therapy for bone metastases. Eur J Nucl Med 1993; 20: 66–74.
Clarke SEM. Radionuclide therapy in oncology. Cancer Treat Rev 1994; 20: 51–71.
Ackery D, Yardly J. Radionuclide-target therapy for the management of metastatic bone pain. Semin Oncol 1993; 20: 27–31.
Turner JH, Claringbold PG, Hetherington EL, Sorby P, Martindale AA. A phase 1 study of samarium-153 ethylenediaminetetramethylene phosphonate therapy for disseminated skeletal metastases. J Clin Oncol 1989; 7: 1926–1931.
Robinson RG, Spicer JA, Preston DF, Wegst AV, Martin NL. Treatment of metastatic bone pain with strontium-89. Nucl Med Biol 1987; 14: 219–222.
Ketring AR. 153Sm-EDTMP and 186Re-HEDP as bone therapeutic radiopharmaceuticals. Nucl Med Biol 1987; 14: 223–232.
Holmes RA. Radiopharmaceuticals in clinical trials. Semin Oncol 1993; 20: 22–26.
Dearnaly DP, Bayly RJ, A'Hern RP, Gadd J, Zivanovic MM, Lewington VJ. Palliation of bone metastases in prostate cancer. Hemibody irradiation or strontium-89? Clin Oncol 1992; 4: 101–107.
Maxon HR, Schroder LE, Thomas SR, Hertzberg VS, Deutsch EA, Scher HI, Samarantunga RC, Libson KF, Williams CC, Moulton JS, Schneider HJ. Re-186(Sn)HEDP for treatment of painful osseous metastases: initial clinical experience in 20 patients with hormone-resistant prostate cancer. Radiology 1990;176:155–159.
de Klerk JMH, Zonnenberg BA, van Rijk PP, van het Schip AD, van Dijk A, Quirijnen JMSP, Looman WJM. Treatment of metastatic bone pain in patients with breast or prostate cancer with Re-186-HEDP. Preliminary results [abstract]. Eur J Nucl Med 1991;18:528
Maxon HR, Schroder LE, Hertzberg VS, Thomas SR, Englaro EE, Samaratunga R, Smith H, Moulton JS, Williams CC, Ehr-hardt GJ, Schneider HJ. Re-186(Sn)HEDP for treatment of painful osseous metastases: results of a double-blind crossover comparison with placebo. J Nucl Med 1991;32:1877–1881.
van het Schip AD, de Klerk JMH, van Dijk A, Zonnenberg BA, van Rijk PP. Pharmacokinetics of Re-186-HEDP: comparison of two formulations in patients with bone metastases [abstract]. Eur J Nucl Med 1993;20:876.
Cancer Therapy Evaluation Program, Division of Cancer Treatments. Common toxicity criteria: guidelines for reporting of adverse drug reactions. Berthesda, Md.: National Cancer Institute, 1988
Maxon HR, Thomas SR, Hertzberg VS, Schroder LE, Englaro EE, Samarantunga R, Scher HI, Moulton JS, Deutsch EA, Deutsch KF, Schneider HJ, Williams CC, Ehrhardt GJ. Rhenium-186 hydroxyethylidene diphosphonate for the treatment of painful bone metastases. Semin Nucl Med 1992;22: 33–40.
Fowler JF. Radiobiological aspects of low dose rates in radioimmunotherapy. Int J Radiat Oncol Biol Phys 1990;18: 1261–1269.
Langmuir VK, Fowler JF, Knox SJ, Wessels BW, Sutherland RM, Wong JYC. Radiobiology of radiolabeled antibody therapy as applied to tumor dosimetry. Med Phys 1993;20: 601–610
Langmuir VK. Radioimmunotherapy: clinical results and dosimetric considerations. Nucl Med Biol 1992;19:213–225.
Sharkey RM, Blumenthal RD, Hansen HJ, et al. Biological considerations for radioimmunotherapy. Cancer Res 1990;50: 964s–969s.
Knox SJ, Goris ML, Wessels BW. Overview of animals studies comparing radioimmunotherapy with dose equivalent external beam irradiation. Radiother Oncol 1992;23:111–117.
de Klerk JMH, van Dijk A, van bet Schip AD, Zonnenberg BA, van Rijk PP. Pharmacokinetics of rhenium- 186-HEDP after administration of rhenium-186-HEDP to patients with bone metastases. J Nucl Med 1992;33:646–651.
Gallini R, Hendry JH, Molineux G, Testa NG. The effect of low dose rate on recovery of hemopoietic and stromal progenitor cells in γ-irradiated mouse bone marrow. Radiat Res 1988; 115:481–487.
de Klerk JMH, van bet Schip AD, Zonnenberg BA, van Dijk A, van Rijk PP. Evaluation of thrombocytopenia in patients treated with Re-186-HEDP: guidelines for individual dose recommendations [abstract]. Eur J Nucl Med 1993;20:987.
Bates T. A review of local radiotherapy in the treatment of bone metastases and cord compression. Int J Radiat Oncol Biol Phys 1992;23:217–221.
Porter AT, McEwan AJB, Powe JE, Reid R, McGowan DG, Lukka H, Sathyanarayana JR, Yakemchuk VN, Thomas GM, Erlich LE, Crook J, Gulenchyn KY, Hong KE, Wesolowsky C, Yardley J. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 1993; 25:805–813.
Fossa SD, Paus E, Lochoff, Melbye Backe S, Aas M. 89Stron-tium in bone metastases from hormone resistant prostate cancer: palliation effect and biochemical changes. Br J Cancer 1992;66:177–180.
Spencer RP. Short lived radionuclides in therapy. Nucl Med Biol 1987; 14:537–538.
Mausner LF, Srivastava SC. Selection of radionuclides for radioimmunotherapy. Med Phys 1993;20:503–509.
Maxon HR, Deutsch EA, Thomas SR, Libson K, Lukes SJ, Williams CC, Ali S. Re- 186(Sn)HEDP for treatment of multiple metastatic foci in bone: human biodistribution and dosimetric studies. Radiology 1988;166:501–507.
Mertens WC. Radionuclide therapy of bone metastases: prospects for enhancement of therapeutical efficacy. Semin Oncol 1993;20:49–55.
Herodin F, Mestries JC, Janodet D, Martin S, Mathieu J, Gascon MP, Pernin MO, Ythier A. Recombinant glycosylated human interleukin-6 accelerates peripheral blood platelet count recovery in radiation-induced bone marrow depression in baboons. Blood 1992;80:688–695.
Zeidler C, Kanz L, Hurkuck F, Rittmann KL, Wildfang I, Kadoya T, Mikayama T, Souza L, Welte K. In vivo effects of interleukin-6 on thrombopoiesis in healthy and irradiated primates. Blood 1992;80:2740–2745.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Klerk, J.M.H., Zonnenberg, B.A., van het Schip, A.D. et al. Dose escalation study of rhenium-186 hydroxyethylidene diphosphonate in patients with metastatic prostate cancer. Eur J Nucl Med 21, 1114–1120 (1994). https://doi.org/10.1007/BF00181067
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00181067