Abstract
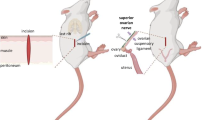
Rats were subjected to right side dorsal rhizotomy of spinal nerves T12-L2 and ipsior contralateral unilateral ovariectomy at estrus (day 1). Estrous cycles were recorded daily, and on day 15 the remaining ovary was removed and weighed. Antral follicles were counted in representative day 1 and day 15 ovaries. Dorsal rhizotomy did not affect estrous cycles during the period after surgery. Also dorsal rhizotomy did not alter ipsilateral ovarian hypertrophy or follicular activation following unilateral ovariectomy. Thus, although the major portion of the ovarian sensory innervation is via the lesioned segments, interruption of these segments centrally does not alter compensatory ovarian responses subsequent to unilateral ovariectomy.
Similar content being viewed by others
References
Arai H (1920) On the cause of the hypertrophy of the surviving ovary after semi-spaying (albino rat) and on the number of ova in it. Am J Anat 28:59–79
Aron M, Aron C, Marescaus J (1948) Les facteurs du fonctionnement ovarien. Recherches de morphologie expérimentale sur certains facteurs intrinseques de ce fonctionnement. C R Assoc Anat 35:10–47
Burden HW, Lawrence IE Jr (1977) The effect of denervation on compensatory ovarian hypertrophy. Neuroendocrinology 23:368–378
Burden HW, Leonard M, Smith CP, Lawrence IE Jr (1983) The sensory innervation of the ovary: a horseradish peroxidase study in the rat. Anat Rec 207:623–627
Burden HW, Lawrence IE Jr, Smith CP, Hoffman J, Leonard M, Fletcher DJ, Hodson CA (1986) The effects of vagatomy on compensatory ovarian hypertrophy and follicular activation after unilateral ovariectomy. Anat Rec 214:61–66
Chavez R, Cruz ME, Dominguez R (1987) Differences in the ovulation rate of the right or left ovary in unilaterally ovariectomized rats: effect of ipsiand contralateral vagus nerves on the remaining ovary. J Endocrinol 113:397–401
DeGreef WJ, Dullaart J, Zeilmaker GH (1975) Serum luteinizing hormone, follicle-stimulating hormone, prolactin and progesterone concentrations and follicular development in the pseudopregnant rat after unilateral ovariectomy. J Endocrinol 66:249–256
Fukuda M, Yamanouchi K, Nakano Y, Furuya H, Arai Y (1984) Hypothalamic laterality in regulating gonadotropic function: unilateral hypothalamic lesion and ovarian compensatory hypertrophy. Neurosci Lett 51:365–370
Gerendai I (1980) Unilateral complete isolation of the medial basal hypothalamus interferes with the compensatory ovarian growth following unilateral ovariectomy. Neuroendocrinol Lett 2:239–243
Gerendai I, Halasz B (1978) Neural participation in ovarian control. Trends Neurosci 1:87–88
Gerendai I, Nemeskeri A (1983) The effect of unilateral vagotomy on compensatory ovarian hypertrophy and on the onset of puberty. In: Endoczi E, Angelluci L, Scapagnini U, deWied D (eds) Neuropeptides, neurotransmitters, and regulation of endocrine processes. Akademiai Kiado, Budapest, pp 191–198
Gerendai I, Marchetti B, Maugeri S, Amico-Roxas M, Scapagnini U (1978) Prevention of compensatory ovarian hypertrophy by local treatment of the ovary with 6-OHDA. Neuroendocrinology 27:272–278
Hatai S (1913) The effect of castration, spaying or semi-spaying on the weight of the central nervous system and of the hypophysis of the albino rat; also the effect of semi-spaying on the remaining ovary. J Exp Zool 15:297–314
Inyama CO, Wharton J, Su HC, Polak JM (1986) CGRP-immunoreactive nerves in the genitalia of the female rat originate from dorsal root ganglia T11 — L3 and L6 — S1: A combined immunocytochemical and retrograde tracing study. Neurosci Lett 69:13–18
Klein CM, Burden HW (1988) Anatomical localization of afferent and postganglionic sympathetic neurons innervating the rat ovary. Neurosci Lett 85:217–222
Nance DM, White JP, Moger WH (1983) Neural regulation of the ovary: evidence for hypothalamic asymmetry in endocrine control. Brain Res Bull 10:353–355
Nance DM, Bhargava M, Myatt GA (1984) Further evidence for hypothalamic asymmetry in endocrine control of the ovary. Brain Res Bull 13:651–655
Nance DM, Burns J, Klein CM, Burden HW (1988) Afferent fibers in the reproductive system and pelvic viscera of female rats: anterograde tracing and immunocytochemical studies. Brain Res Bull 21:701–709
Peppler RD (1971) Effects of unilateral ovariectomy on follicular development and ovulation in cycling, aged rats. Am J Anat 132:423–428
Peppler RD, Greenwald GS (1970) Influence of unilateral ovariectomy on follicular development in cycling rats. Am J Anat 127:9–14
Ramirez VD, Sawyer CH (1974) A sex difference in the rat pituitary FSH response to unilateral gonadectomy as revealed in plasma radioimmunoassays. Endocrinology 94:475–482
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burden, H.W., Price, G.T., Davenport, A.T. et al. Dorsal rhizotomy does not alter rat estrous cycles or ovarian compensatory responses. Anat Embryol 187, 461–464 (1993). https://doi.org/10.1007/BF00174421
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00174421