Summary
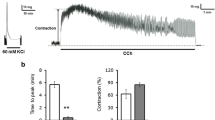
The contraction of longitudinal muscle strips of the rabbit duodenum in response to motilin and acetylcholine was investigated in normal and high K+-solutions in the presence and absence of external calcium, in order to demonstrate the existence of pharmaco-mechanical coupling for motilin and to examine whether the peptide mobilizes calcium from an intracellular store. In depolarized smooth muscle (140 mM K+), motilin (3.2×109 −1×10−7 M) and acetylcholine (1×10−5 M) were still capable of causing a considerable, transient, concentration-dependent contraction in the presence of Ca2+. The ‘extra’-contraction to motilin was not blocked by tetrodotoxin (1 μg/ml) nor by atropine (10−7 M), but acetylcholine (10−5 M) was blocked by atropine. Verapamil (10−7 M) could selectively block the K+ contraction without affecting the extra agonist contraction. Nitroprusside was ineffective up to 10−4 M in high K+-solutions, but in normal Hepes-buffer it caused a concentration-dependent rightward shift of the concentration-response curve of motilin and acetylcholine contractions. In a calcium-depleted medium, high K+-depolarized muscle strips were still responsive to motilin and acetylcholine, but higher concentrations (10−6 M) were needed than in the presence of calcium and the contractions reached only 57 +- 11% and 74 +- 9% respectively of the maximal contraction in 1.2 mM Ca2+ containing solutions. The response to motilin (10−6 M) was not only smaller than that to acetylcholine (10−5 M), it also faded more rapidly with time. The response to one agonist could not be repeated except by using a higher concentration of the same or the other agonist, and the magnitude of this second response depended upon the dose used in the first one. We conclude that pharmaco-mechanical coupling exists for motilin and that this peptide is able to elicit contractions by mobilization of calcium from an intracellular store. This store overlaps with the one used by acetylcholine. Our experiments also reinforce the hypothesis that in the rabbit motilin exerts a direct action upon smooth muscle cells.
Similar content being viewed by others
References
Adachi H, Toda H, Hayashi S, Noguchi M, Suzuki T, Torizuka K, Yajima H, Koyama K (1981) Mechanism of the excitatory action of motilin on isolated rabbit intestine. Gastroenterology 80:783–788
Ambache N (1954) Separation of the longitudinal muscle of the rabbit's ileum as a broad sheet. J Physiol 125:53–54
Berridge MJ (1984) Inositol trisphosphate and diacylglycerol as second messengers. Biochem J 220:345–360
Bond M, Kitazawa T, Somlyo AP, Somlyo AV (1984) Release and recycling of calcium by the sarcoplasmic recticulum in guinea-pig portal vein smooth muscle. J Physiol 355:677–695
Bormans V, Peeters TL, Vantrappen G (1986) Motilin receptors in rabbit stomach and small intestine. Regul Pept 15:143–153
Brown JC, Cook MA, Dryburgh JR (1973) Motilin, a gastric motor activity stimulating polypeptide: the complete amino acid sequence. Can J Biochem 51:533–537
Casteels R, Raeymaekers L (1979) The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol 294:51–68
Domschke W, Strunz V, Mitznegg P, Domschke S, Wünsch E, Demling L (1976) Motilin and motilin analogues: Mode of Action. Scand J Gastroenterol 11, Suppl 39:25–28
Exton JH (1986) Mechanism involved in calcium-mobilizing agonist responses. In: Greengard P, Robinson GA (eds) Advances in cyclic nucleotide and protein phosphorylation research, vol 20. Raven Press, New York, pp 211–262
Gabella G (1987) Structure of muscles and nerves in the gastrointestinal tract. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 2nd edn. Raven Press, New York, pp 335–381
Giembycz MA, Rodger IW (1987) Electrophysiological and other aspects of excitation-contraction coupling and uncoupling in mammalian airway smooth muscle. Life Sci 41:111–132
Hartshorne DJ (1987) Biochemistry of the contractile process in smooth muscle. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 2nd edn. Raven Press, New York, pp 423–482
Himpens B, Somlyo AP (1988) Free calcium transients during electromechanical and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol (Lond) 395:507–530
Himpens B, Matthijs G, Somlyo AV, Butler TM, Somlyo AP (1988) Cytoplasmic free calcium, myosin light chain phosphorylation and force in phasic and tonic smooth muscle. J Gen Physiol 12:713–729
Holzer P, Lippe IT (1984) Substance P can contract the longitudinal muscle of the guinea-pig small intestine by releasing intracellular calcium. Br J Pharmacol 82:259–267
Holzer P, Lippe IT (1985) Substance P action on phosphoinositides in guinea-pig intestinal muscle: a possible transduction mechanism? Naunyn-Schmiedeberg's Arch Pharmacol 326:50–55
Karaki H, Weiss GB (1984) Calcium channels in smooth muscle. Gastroenterology 87:960–970
Karaki H, Sato K, Ozaki H, Murakami K (1988) Effects of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. Eur J Pharmacol 156:259–266
Keatinge WR (1972) Ca concentrations and flux in Ca-deprived arteries. J Physiol 224:35–59
Kobayashi S, Twanaga T, Fujita T, Yanaihara N (1980) Do enterochromaffn (EC) cells contain motilin? Arch Histol Jpn 43:85–98
Matthijs G, Peeters TL, Vantrappen G (1988) Effect of different calcium-modulators on motilin-induced contractions of the rabbit duodenum. Comparison with acetylcholine. Regul Pept 21:321–330
Merritt JE, Rink TJ (1987) The effects of substance P and carbachol on inositol tris- and tetrakisphosphate formation and cytosolic free calcium in rat parotid acinar cells. J Biol Chem 262:14912–14916
Peeters TL, Vantrappen G, Janssens J (1980) Fasting motilin levels are related to the interdigestive motility complex. Gastroenterology 79:716–719
Peeters TL, Bormans V, Vantrappen G (1988) Comparison of motilin binding to crude homogenates of human and canine gastrointestinal smooth muscle tissue. Regul Pept 23:171–182
Rapoport RM, Schwartz K, Murad F (1985) Effect of sodium-potassium pump inhibitors and membrane-depolarizing agents on sodium nitroprusside-induced relaxation and cyclic guanosine monophosphate accumulation in rat aorta. Circ Res 57:164–170
Riemer J, Kölling K, Mayer CJ (1977) The effects of motilin on the electrical activity of rabbit circular duodenal muscle. Pflügers Arch 372:243–250
Somlyo AP, Somlyo AV (1968) Vascular smooth muscle. I. Normal structure, pathology, biochemistry and biophysics. Pharmacol Rev 20:197–272
Somlyo AP (1985) Excitation-contraction coupling and the ultrastructure of smooth muscle. Circ Res 57:497–507
Sommerville LE, Hartshorne DJ (1986) Intracellular calcium and smooth muscle contraction. Cell Calcium 7:353–364
Strunz U, Domschke W, Mitznegg P, Domschke S, Schubert E, Wünsch E, Jaeger E, Demling L (1975) Analysis of the motor effects of 13-norleucine motilin on the rabbit, guinea pig, rat and human alimentary tact in vitro. Gastroenterology 68: 1485–1491
Vantrappen G, Peeters TL (1989) Motilin. In: Makhlouf GM (ed) Handbook of physiology. Endocrinology of the gastrointestinal system. The American Physiological Society (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matthijs, G., Peeters, T.L. & Vantrappen, G. The role of intracellular calcium stores in motilin induced contractions of the longitudinal muscle of the rabbit duodenum. Naunyn-Schmiedeberg's Arch Pharmacol 339, 332–339 (1989). https://doi.org/10.1007/BF00173588
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00173588