Abstract
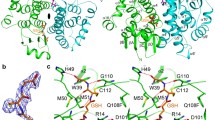
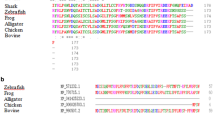
Our previous studies have shown that the S-crystallins of cephalopod (Ommastrephes sloani pacificus) eye lenses comprise a family of at least ten members which are evolutionarily related to glutathione S-transferase (GST, EC 2.5.1.18). Here we show by cDNA cloning that there are at least 24 different S-crystallins that are 46–99% identical to each other by amino acid sequence in the squid Loligo opalescens. In each species, all but one S-crystallin (SL11 in O. pacificus and Lops4 in L. opalescens) examined has an inserted central peptide of variable length and sequence. cDNA expression studies conducted in Escherichia coli showed that squid GST (which is expressed little in the lens) has very high enzymatic activity using 1-chloro-2, 4-dinitrobenzene (CDNB) as a substrate; by contrast, SL20-1 of O. pacificus and Lops 12 of L. opalescens (which are encoded by abundant lens mRNAs) have no GST activity. Interestingly, SL11 and Lops4 have some enzymatic activity with the CDNB substrate. Site-specific mutations at Y7 or W38, both residues essential for activity of vertebrate GSTs, or insertion of the central peptide present in the inactive SL20-1, reduced the specific activity of squid GST by 30- to 100-fold. These data indicate that the S-crystallins consist of a family of enzymatically inactive proteins (when using CDNB as a substrate) which is considerably larger than previously believed and that GST activity was lost by gradual drift in sequence as well as by insertion of an extra peptide by exon shuffling. The results are also consistent with the idea that SL11 and Lops4 are orthologous crystallins representing the first descendants of the ancestral GST gene in the pathway which gave rise to the extensive S-crystallin family of lens proteins.
Similar content being viewed by others
References
Armstrong RN (1991) Glutathione S-transferases: reaction mechanism, structure, and function. Chem Res Toxicol 4:131–140
Armstrong RN (1994) Glutathione S-transferases: structure and mechanism of an archetypical detoxification enzyme. Adv Enzymol Relat Areas Mol Biol 69:1–44
Baker RT, Smith SA, Marano R, McKee J, Board PG (1994) Protein expression using cotranslational fusion and cleavage of ubiquitin. Mutagenesis of the glutathione-binding site of human Pi class glutathione S-transferase. J Biol Chem 269:25381–25386
Chiou S-H (1984) Physicochemical characterization of a crystallin from the squid lens and its comparison with vertebrate lens crystallins. J Biochem (Tokyo) 95:75–82
Cuthbertson RA, Tomarev SI, Piatigorsky J (1992) Taxon-specific recruitment of enzymes as major soluble proteins in the corneal epithelium of three mammals, chicken and squid. Proc Natl Acad Sci USA 89:4004–4008
Cvekl A, Sax CM, Bresnick EH, Piatigorsky J (1994) A complex array of positive and negative elements regulates the chicken αA-crystallin gene: involvement of Pax-6, USF, CREB and/or CREM, and AP-1 proteins. Mol Cell Biol 14:7363–7376
Cvekl A, Kashanchi F, Sax CM, Brady JN, Piatigorsky J (1995a) Transcriptional regulation of the mouse αA-crystallin gene: activation dependent on cyclic AMP-responsive element (DE1/CRE) and a Pax-6-binding site. Mol Cell Biol 15:653–660
Cvekl A, Sax CM, Li X, McDermott JB, Piatigorsky J (1995b) Pax-6 and lens-specific transcription of the chicken δ1-crystallin gene. Proc Natl Acad Sci USA 92:4681–4685
de Jong WW, Hendriks W, Mulders JWM, Bloemendal H (1989) Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci 14:365–368
de Jong WW, Lubsen NH, Kraft HJ (1994) Molecular evolution of the eye lens. Progr Retinal Eye Res 13:391–442
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387–395
Dirr H, Reinemer P, Huber R (1994a) X-ray crystal structures of cytosolic glutathione S-transferases. Implications for protein architecture, substrate recognition and catalytic function. Eur J Biochem 220:645–661
Dirr H, Reinemer P, Huber R (1994b) Refined crystal structure of porcine class pi glutathione S-transferase (pGST Pl-1) at 2.1 A resolution. J Mol Biol 243:72–92
El Hawrani AS, Moreton KM, Sessions RB, Clarke AR, Holbrook JJ (1994) Engineering surface loops of proteins—a preferred strategy for obtaining new enzyme function. Trends Biotech 12:207–211
Felsenstein J (1993) PHYLIP (phylogeny inference package) version 3.5c. Department of Genetics, University of Washington, Seattle
Gross M, Jaenicke R (1994) Proteins under pressure. The influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur J Biochem 221:617–630
Habig WB, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first step in mercapturic acid formation. J Biol Chem 249: 7130–7139
Halder G, Callaerts P, Gehring WJ (1995) Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267: 1788–1792
Harris J, Coles B, Meyer DJ, Ketterer B (1991) The isolation and characterization of the major glutathione S-transferase from the squid Loligo vulgaris. Comp Biochem Physiol 98B:511–515
Hill RE, Favor J, Hogan BLM, Ton CCT, Saunders GF, Hanson IM, Prosser J, Hastie ND, van Heynigen VV (1991) Mouse small eye results from mutations in paired-like homeobox-containing gene. Nature 354:522–525
Ho SN, Hunt HD, Horton RM, Pollen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59
Ji X, Zhang P, Armstrong RN, Gilliland GL (1992) The three-dimensional structure of a glutathione S-transferase from the mu gene class. Structural analysis of the binary complex of isoenzyme 3–3 and glutathione at 2.2-A resolution. Biochemistry 31:10169–10184
Ji X, Johnson WW, Sesay MA, Dickert L, Prasad SM, Ammon HL, Armstrong RN, Gilliland GL (1994) Structure and function of the xenobiotic substrate binding site of a glutathione S-transferase as revealed by X-ray crystallographic analysis of product complexes with the diastereomers of 9-(S-glutathionyl)-10-hydroxy-9,10-dihydrophenanthrene. Biochemistry 33:1043–1052
Ji X, von Rosenvinge EC, Johnson WW, Tomarev SI, Piatigorsky J, Armstrong RN, Gilliland GL (1995) Three-dimensional structure, catalytic properties and evolution of a sigma class glutathione transferase from squid, a progenitor of the lens S-crystallins of cephalopods. Biochemistry 34:5317–5328
Jones DH, Howard BH (1990) A rapid method for site-specific mutagenesis and directional subcloning by using the polymerase chain reaction to generate recombinant circles. Biotechnique 8:178–183
Johnson WW, Liu S, Ji X, Gilliland GL, Armstrong RN (1993) Tyrosine 115 participates both in chemical and physical steps of the catalytic mechanism of a glutathione S-transferase. J Biol Chem 268:11508–11511
Kohn RH, Sroga GE, Mannervik B (1992) Participation of the phenolic hydroxyl group of Tyr-8 in the catalytic mechanism of human glutathione transferase Pl-1. Biochem J 285:537–540
Kong K-H, Nishida M, Inoue H, Takahashi K (1992) Tyrosine-7 is an essential residue for the catalytic activity of human class Pi glutathione S-transferase: chemical modification and site-directed mutagenesis studies. Biochem Biophys Res Common 182:1122–1129
Land MF, Fernald RD (1992) The evolution of eyes. Annu Rev Neurosci 15:1–29
Liu S, Zhang P, Ji X, Johnson WW, Gilliland GL, Armstrong RN (1992) Contribution of tyrosine 6 to the catalytic mechanism of isoenzyme 3–3 of glutathione S-transferase. J Biol Chem 267:4296–4299
Nishihira J, Ishibashi T, Sakai M, Nishi S, Kumazaki T (1992) Evidence for the involvement of tryptophan 38 in the active site of glutathione S-transferase P. Biochem Biophys Res Common 185: 1069–1077
Olds RJ, Lane DA, Chowdhury V, De Stefano V, Leone G, Them SL (1993) Complete nucleotide sequence of the antithrombin gene: evidence for homologous recombination causing thrombophilia. Biochemistry 32:4216–4224
Packard A (1972) Cephalopods and fish: the limits of convergence. Biol Rev 47:241–307
Piatigorsky J (1992) Innovation associated with changes in gene regulation. J Biol Chem 267:4277–4280
Piatigorsky J, Wistow G (1989) Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell 57:197–199
Piatigorsky J, Wistow G (1991) The recruitment of crystallins: new functions precede gene duplication. Science 252:1078–1079
Piatigorsky J, Zelenka PS (1992) Transcriptional regulation of crystallin genes: cis elements, trans-factors, and signal transduction systems in the lens. Adv Dev Biochem 1:211–256
Quiring R, Walldorf U, Kloter U, Gehring WJ (1994) Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265:785–789
Reinemer P, Dirr HW, Ladenstein R, Schaffer J, Gallay O, Huber R (1991) The three-dimensional structure of class π glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J 10:1997–2005
Richardson J, Cvekl A, Wistow G (1995) Pax-6 is essential for lens-specific expression of ζ-crystallin. Proc Natl Acad Sci USA 92:4676–4680
Siezen RJ, Shaw DC (1982) Physicochemical characterization of lens proteins of the squid Nototodarus gouldi and comparison with vertebrate crystallins. Biochim Biophys Acta 704:304–320
Spector A (1991) The lens and oxidative stress. In: Sies H (ed) Oxidative stress: oxidants and antioxidants. Academic Press, New York, pp 529–558
Stenberg G, Board PG, Mannervik B (1991) Mutation of an evolutionary conserved tyrosine residue in the active site of human class alpha glutathione transferase. FEBS Lett 293:153–155
Stoppa-Lyonnet D, Carter PE, Meo T, Tosi M (1990) Cluster of intragenic Alu repeats predispose the human C1 inhibitor locus to deleterious rearrangement. Proc Natl Acad Sci USA 87:1551–1555
Tang S-S, Lin C-C, Chang,G-G (1994) Isolation and characterization of octopus hepatopancreatic glutathione S-transferase. Comparison of digestive gland enzyme with lens S-crystallin. J Protein Chem 13:609–618
Tomarev SI, Zinovieva RD (1988) Squid major lens polypeptides are homologous to glutathione S-transferase subunits. Nature 336:86–88
Tomarev SI, Zinovieva RD, Piatigorsky J (1991) Crystallins of the octopus lens. Recruitment from detoxification enzymes. J Biol Chem 266:24226–24231
Tomarev SI, Zinovieva RD, Piatigorsky J (1992) Characterization of squid crystallin genes. Comparison with mammalian glutathione S-transferase genes. J Biol Chem 267:8604–8612
Tomarev SI, Zinovieva RD, Guo K, Piatigorsky J (1993) Squid glutathione S-transferase. Relationships with other glutathione S-transferases and S-crystallins of cephalopods. J Biol Chem 268:4534–4542
Tomarev SI, Duncan MK, Roth HJ, Cvekl A, Piatigorsky J (1994) Convergent evolution of crystallin gene regulation in squid and chicken: the AP-1/ARE connection. J Mol Evol 39:134–143
Ton CCT, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, Royer-Pokora B, Collins F, Swaroop A, Strong LC, Saunders GF (1991) Positional cloning and characterization of a paired box- and homeobox-containing gene from aniridia region. Cell 67:1059–1074
Wang RW, Newton DJ, Huskey S-EW, McKeever BM, Pickett CB, Lu AYH (1992) Site-directed mutagenesis of glutathione S-transferase YaYa. Important roles of tyrosine 9 and aspartic acid 101 in catalysis. J Biol Chem 267:19866–19871
Wilce MCJ, Parker MW (1994) Structure and function of glutathione S-transferases. Biochim Biophys Acta 1205:1–18
Wistow G, Piatigorsky J (1987) Recruitment of enzymes as lens structural proteins. Science 236:1554–1556
Wistow G, Piatigorsky J (1988) Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem 57:479–504
Wistow G (1993) Lens crystallin: gene recruitment and evolutionary dynamism. Trends Biochem Sci 18:301–306
Zuckerkandl E (1994) Molecular pathways to parallel evolution: I. Gene nexuses and their morphological correlates. J Mol Evol 39: 661–678
Zuker CS (1994) On the evolution of eyes: would you like it simple or compound? Science 265:742–743
Author information
Authors and Affiliations
Additional information
Correspondence to: S.I. Tomarev
Rights and permissions
About this article
Cite this article
Tomarev, S.I., Chung, S. & Piatigorsky, J. Glutathione S-transferase and S-crystallins of cephalopods: Evolution from active enzyme to lens-refractive proteins. J Mol Evol 41, 1048–1056 (1995). https://doi.org/10.1007/BF00173186
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00173186